Question
In the first step of glycolysis, the following two reactions are coupled: Reaction 1: glucose + P glucose-6-phosphate + HO AG +13.8 kJ/mol AG=
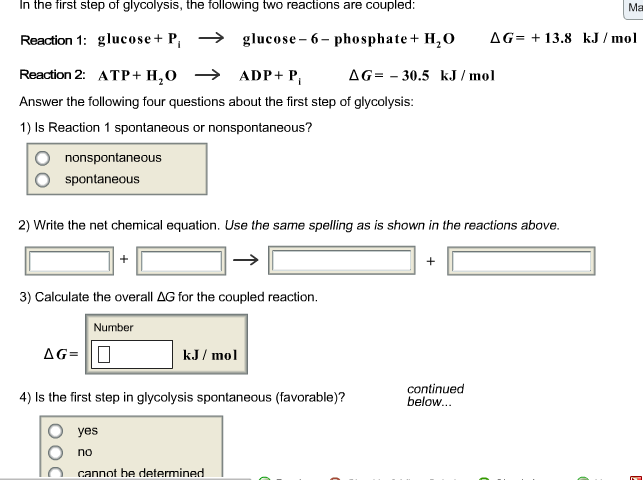
In the first step of glycolysis, the following two reactions are coupled: Reaction 1: glucose + P glucose-6-phosphate + HO AG +13.8 kJ/mol AG= 30.5 kJ/mol Reaction 2: ATP + HO ADP + P Answer the following four questions about the first step of glycolysis: 1) Is Reaction 1 spontaneous or nonspontaneous? nonspontaneous spontaneous 2) Write the net chemical equation. Use the same spelling as is shown in the reactions above. 3) Calculate the overall AG for the coupled reaction. AG= Number kJ/mol 4) Is the first step in glycolysis spontaneous (favorable)? yes no cannot be determined continued below... Ma 3
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION The change in free energy associated with a certain reaction is refe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Biochemistry Concepts and Connections
Authors: Dean R. Appling, Spencer J. Anthony Cahill, Christopher K. Mathews
1st edition
321839927, 978-0133853490, 133853497, 978-0321839923
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



