Question
In the following process for condensing methanol vapor from air, most of the entering methanol is liquefied in this steady state process, with the remaining
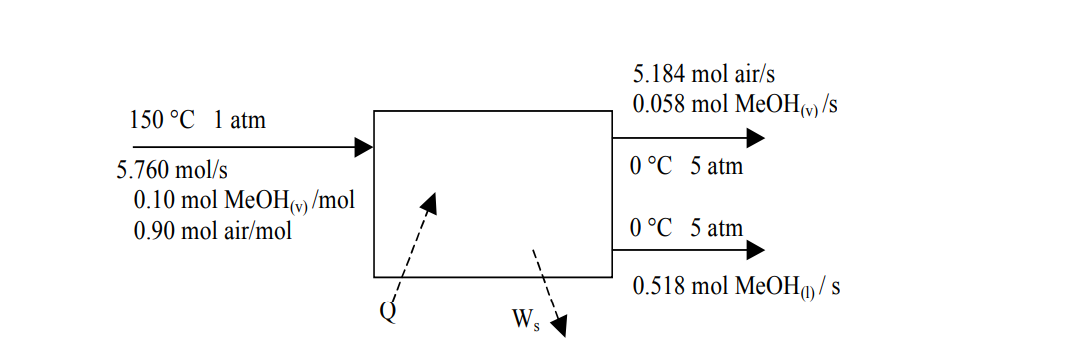
In the following process for condensing methanol vapor from air, most of the entering methanol is liquefied in this steady state process, with the remaining frac:on exi:ng with the air stream. Both exit streams are at 0o C and 5 atm. Shaft work is delivered to the system at a rate of 30 kW to achieve the compression. Construct an inlet-outlet enthalpy table for the process, and calculate all unknown enthalpies. Iden:fy the reference states selected for the components, and write all assump:ons. What is the rate at which heat must be removed from the condenser (kW)?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


