Answered step by step
Verified Expert Solution
Question
1 Approved Answer
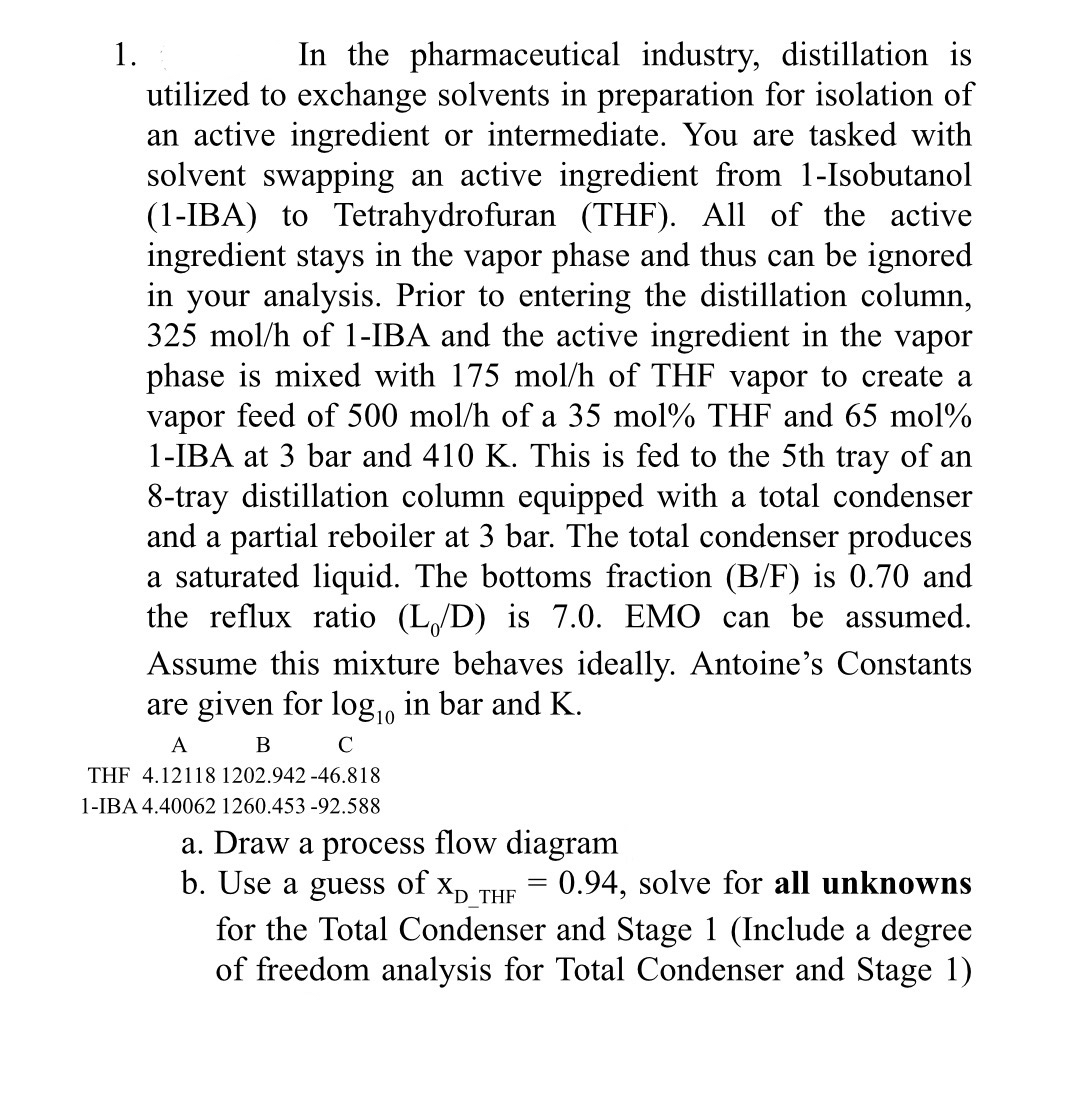
In the pharmaceutical industry, distillation is utilized to exchange solvents in preparation for isolation of an active ingredient or intermediate. You are tasked with solvent
In the pharmaceutical industry, distillation is utilized to exchange solvents in preparation for isolation of an active ingredient or intermediate. You are tasked with solvent swapping an active ingredient from Isobutanol IBA to Tetrahydrofuran THF All of the active ingredient stays in the vapor phase and thus can be ignored in your analysis. Prior to entering the distillation column, of IBA and the active ingredient in the vapor phase is mixed with of THF vapor to create a vapor feed of of a mol THF and molIBA at bar and This is fed to the th tray of an tray distillation column equipped with a total condenser and a partial reboiler at bar. The total condenser produces a saturated liquid. The bottoms fraction is and the reflux ratio is EMO can be assumed. Assume this mixture behaves ideally. Antoine's Constants are given for in bar and
THF
IBA
a Draw a process flow diagram
b Use a guess of solve for all unknowns for the Total Condenser and Stage Include a degree of freedom analysis for Total Condenser and Stage
Part b plz
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


