Question
In the process of humidification operation, air with a relative humidity = 20% and temperature = 130 oF requires 15,000 lb/hr based on dry air.
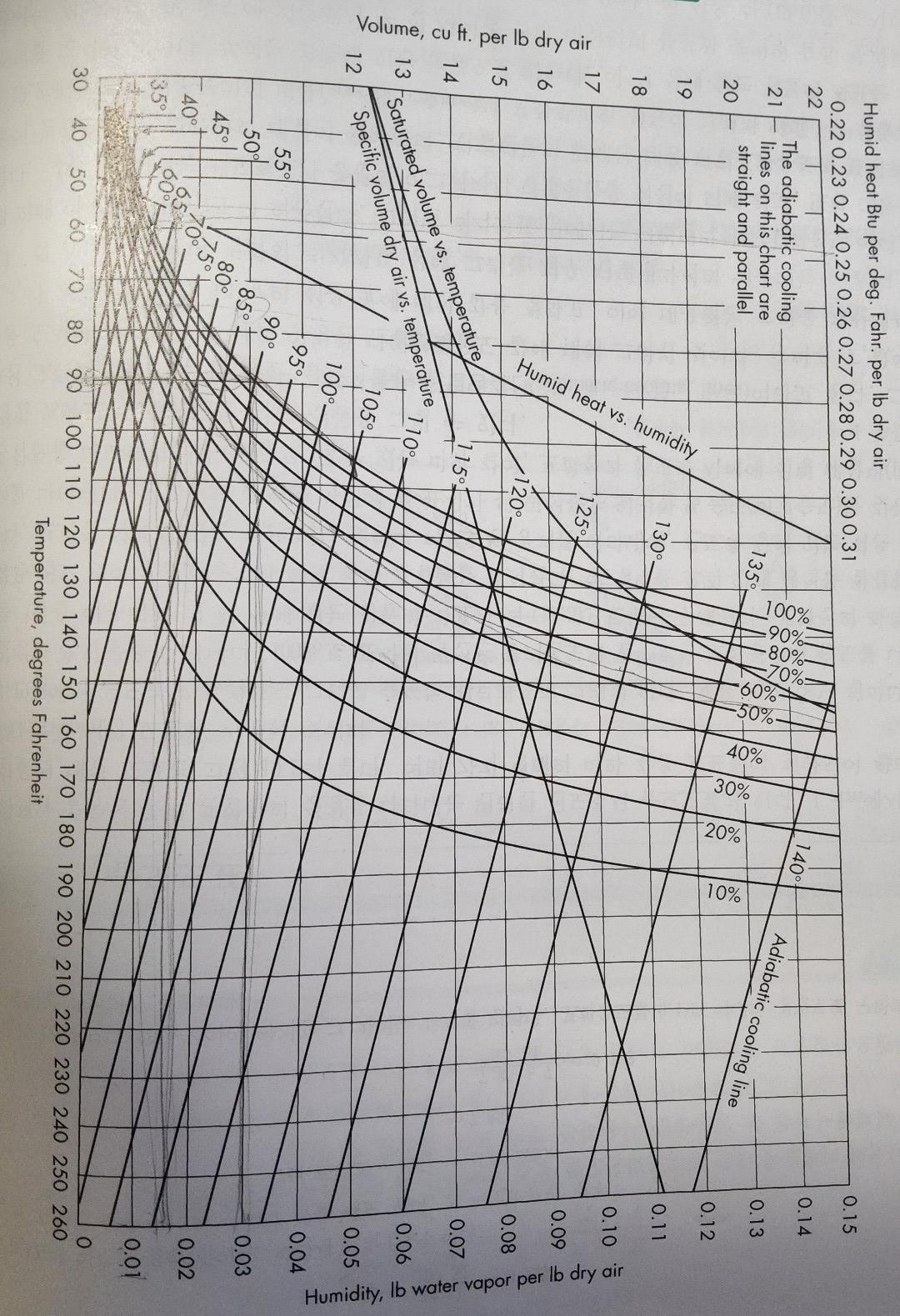
In the process of humidification operation, air with a relative humidity = 20% and temperature = 130 oF requires 15,000 lb/hr based on dry air. Air in this condition can be obtained by first vaporizing air with a humidity of 20% and a temperature of 70 Fahrenheit to the required humidity adiabatically, and finally heating the vaporized air to a temperature of 130 Fahrenheit again. The vaporizing step is carried out in the spray chamber. The air leaving the spray chamber is 4 degrees Fahrenheit higher than the adiabatic saturation temperature. ( hya = 85 Btu/ft3*hr*oF, cs(70oF) = 0.241 Btu /lb*oF, cs(850F) = 0.250 Btu/lb*oF) Answer next a)Air preheating temperature b) Release temperature of air from the spray chamber c) Heat required for preheating (Btu/hr) d) Heat required for reheating e)Volume of spray chamber (ft3)

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


