Question
In the process sketched in Figure, Na2CO3 is produced by the reaction Na2S + CaCO3 Na2CO3 + CaS. The reaction is 90% complete on

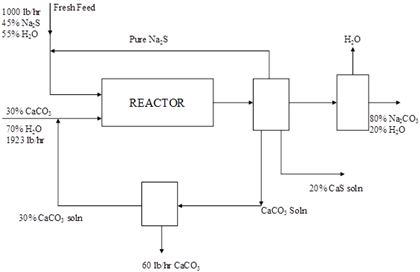
In the process sketched in Figure, Na2CO3 is produced by the reaction Na2S + CaCO3 Na2CO3 + CaS. The reaction is 90% complete on one pass through the reactor, and the amount of CaCO3 entering the reactor is 50% in excess of that needed. Calculate on the basis of 1000 lb/hr of fresh feed (a) the pounds of Na2S recycled, and (b) the pounds of Na2CO3 solution formed per hour. 1000 lb/hr 45% Na S 55% HO 30% CaCO 70% HO 1923 lb hr Fresh Feed 30% CaCO, soln Pure Na $ REACTOR 60 lb/hr CaCO, CaCO, Soln HO 20% CaS soln $0% NaCO 20% HO
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Chemical Principles
Authors: Steven S. Zumdahl, Donald J. DeCoste
7th edition
9781133109235, 1111580650, 978-1111580650
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



