Answered step by step
Verified Expert Solution
Question
1 Approved Answer
In the production of aniline by the hydrogenation of nitrobenzene, the reactor products are separated from unreacted hydrogen in a condenser. The condensate, which is
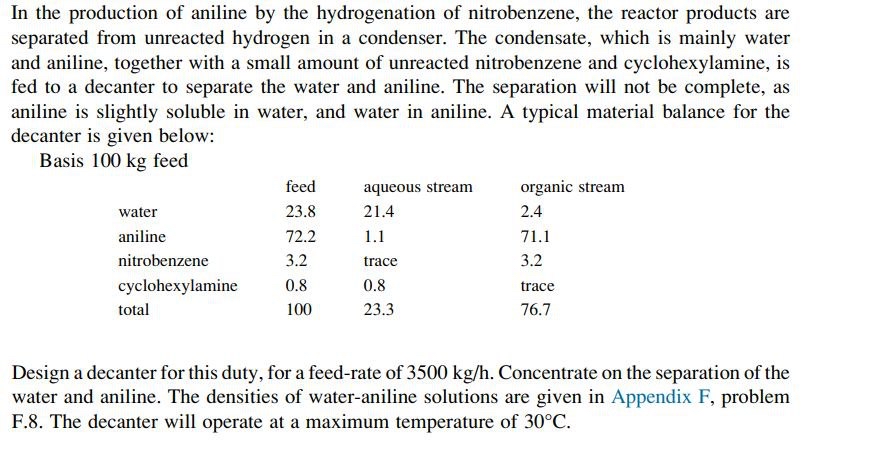
In the production of aniline by the hydrogenation of nitrobenzene, the reactor products are separated from unreacted hydrogen in a condenser. The condensate, which is mainly water and aniline, together with a small amount of unreacted nitrobenzene and cyclohexylamine, is fed to a decanter to separate the water and aniline. The separation will not be complete, as aniline is slightly soluble in water, and water in aniline. A typical material balance for the decanter is given below:
Basis feed
tablefeed,aqueous stream,organic streamwateranilinenitrobenzenetrace,cyclohexylaminetracetotal
Design a decanter for this duty, for a feedrate of Concentrate on the separation of the water and aniline. The densities of wateraniline solutions are given in Appendix F problem F The decanter will operate at a maximum temperature of
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


