Answered step by step
Verified Expert Solution
Question
1 Approved Answer
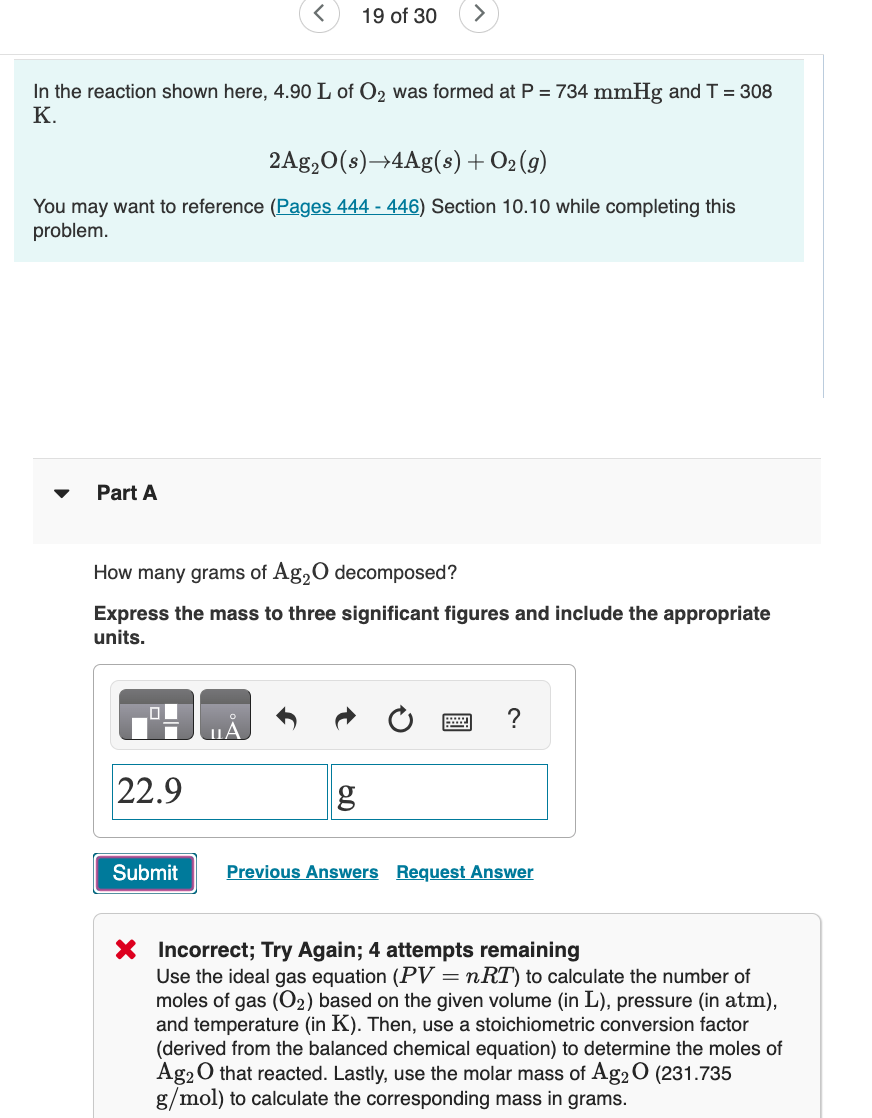
In the reaction shown here, 4 . 9 0 L of O 2 was formed at P = 7 3 4 m m H g
In the reaction shown here, of was formed at and
You may want to reference Pages Section while completing this
problem.
Part A
How many grams of decomposed?
Express the mass to three significant figures and include the appropriate
units.
Previous Answers
Incorrect; Try Again; attempts remaining
Use the ideal gas equation to calculate the number of
moles of gas based on the given volume in pressure in atm
and temperature in Then, use a stoichiometric conversion factor
derived from the balanced chemical equation to determine the moles of
that reacted. Lastly, use the molar mass of
to calculate the corresponding mass in grams.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


