Question
In thermodynamics, the equilibrium state of a system is the one that maximizes the total entropy out of the variety of states that are compatible
In thermodynamics, the equilibrium state of a system is the one that maximizes the total entropy out of the variety of states that are compatible with the constraints. We assume that the entropy S is additive over disjoint subsystems and also that the partial derivative of S with respect to the energy E is strictly positive (i.e., the temperature T > 0). The latter allows one to express E as a function of S and the other extensive parameters, e.g. E = E(S, V, N) where V is the volume and N the total number of particles. One can then express the first law of thermodynamics in differential form as dE = T dS pdV + dN, where p and are the pressure and chemical potential, respectively. This gives a way to define the intensive parameters in terms of derivatives of the energy:
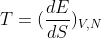
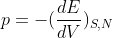
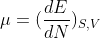
a.) Show that S is a first-order homogenous function of its extensive parameters and use that property to derive Euler's relation TS = E + PV - 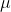 N. Derive also the Gibbs-Duhem relation
N. Derive also the Gibbs-Duhem relation 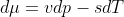 , where v = V/N and s = S/N are the volume and entropy per particle.
, where v = V/N and s = S/N are the volume and entropy per particle.
b.) Use the properties discussed above to show that S is a concave function of E.
de T= Gav, N ds de E p p=-G), = d NStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


