Answered step by step
Verified Expert Solution
Question
1 Approved Answer
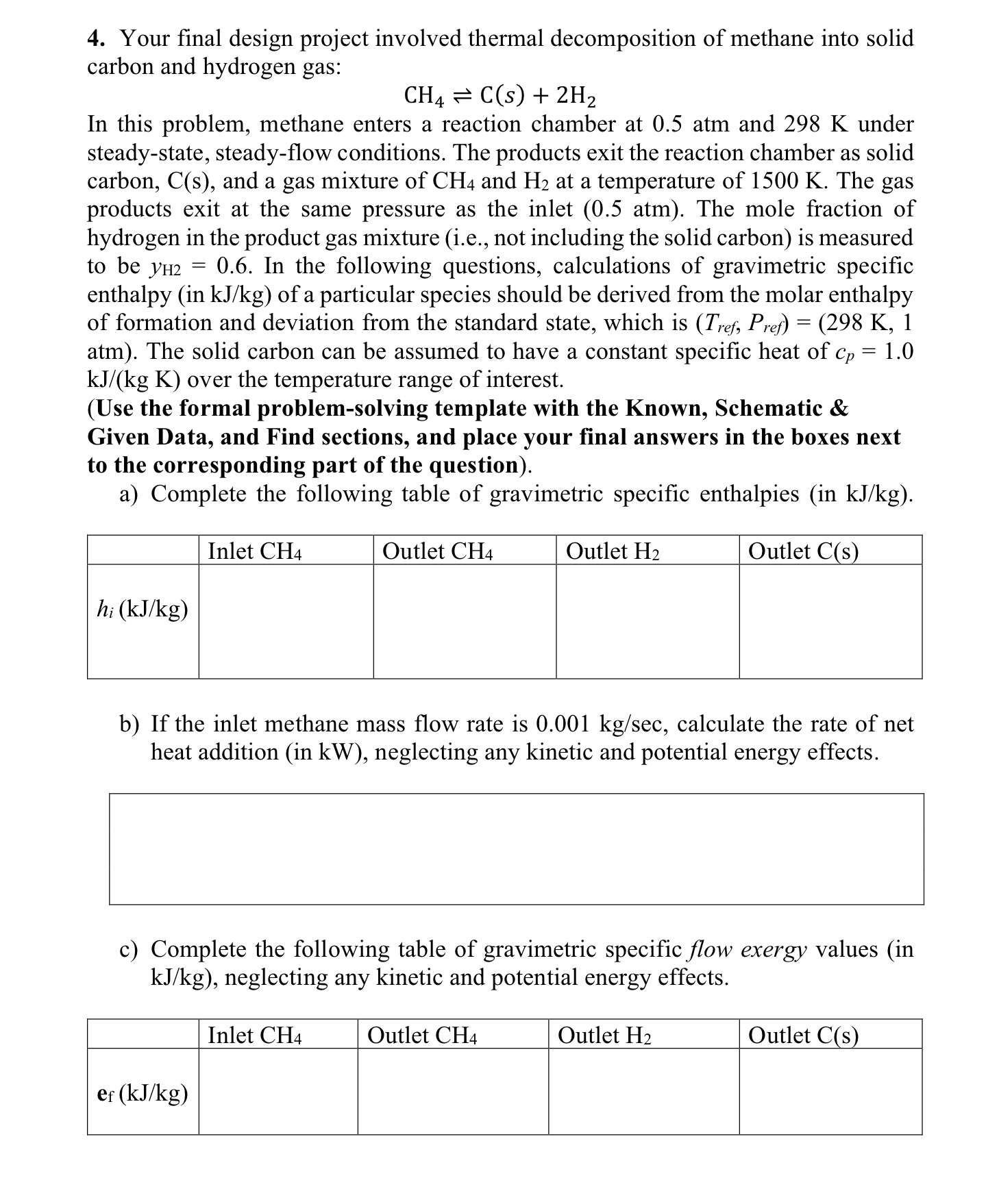
In this problem, methane enters a reaction chamber at 0 . 5 atm and 2 9 8 K under steady - state, steady - flow
In this problem, methane enters a reaction chamber at atm and under
steadystate, steadyflow conditions. The products exit the reaction chamber as solid
carbon, and a gas mixture of and at a temperature of The gas
products exit at the same pressure as the inlet atm The mole fraction of
hydrogen in the product gas mixture ie not including the solid carbon is measured
to be In the following questions, calculations of gravimetric specific
enthalpy in of a particular species should be derived from the molar enthalpy
of formation and deviation from the standard state, which is
atm The solid carbon can be assumed to have a constant specific heat of
over the temperature range of interest.
Use the formal problemsolving template with the Known, Schematic &
Given Data, and Find sections, and place your final answers in the boxes next
to the corresponding part of the question
a Complete the following table of gravimetric specific enthalpies in
b If the inlet methane mass flow rate is calculate the rate of net
heat addition in neglecting any kinetic and potential energy effects.
c Complete the following table of gravimetric specific flow exergy values in
neglecting any kinetic and potential energy effects.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


