Question
In this problem, we intend to analyse the impact of the discharge of a treated Urban Waste Water (UWW) in an unpolluted river (see Figures
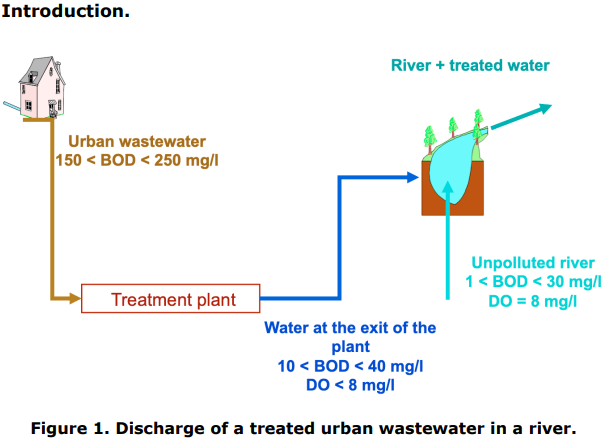
In this problem, we intend to analyse the impact of the discharge of a treated Urban Waste Water (UWW) in an unpolluted river (see Figures 1 and 2). Usually, untreated UWW has a Biological Oxygen Demand (BOD) between 150 and 250 mg/l. After treatment, the goal is usually to have water with a BOD between 10 and 40 mg/l. As the biological treatment of an UWW consumes oxygen, the water going out of the biological treatment has a dissolved oxygen concentration (denoted DO) lower than the saturation concentration. Consequently, a final re-oxygenation treatment of an UWW is usually carried out in order to increase the concentration of dissolved oxygen to a value close to saturation before delivery in the aquatic environment. In this problem, we assume that such a re-oxygenation is realized, yielding a treated UWW saturated with oxygen. Unpolluted surface waters generally have a BOD between 1 and 30 mg/l and are often saturated with oxygen.
The impact of the discharge of a treated UWW in an unpolluted river is shown schematically in Figure 2. A z coordinate is introduced. z measures the distance from the discharge point in the river. After discharge and in the river, the microorganisms naturally present in the river and in the treated water degrade the organic matter. The rate of degradation of the organic matter at a position z in the river, written rBOD(z) (in mg of BOD consumed per litre of river and per day), is assumed to be of the first order with respect to the concentration of organic matter: rBOD(z) = k1BOD(z), with BOD(z) the biological oxygen demand of the water in the river at position z. This explains the exponential decrease of BOD(z) presented in Figure 2.
Oxygen consumption by the microorganisms goes along this biodegradation. According to the definition of the BOD, the rate of oxygen consumption by the microorganisms at position z in the river, written rDO(z) (in mg of oxygen consumed per litre of river and per day), is equal to rBOD(z).
As soon as oxygen consumption starts, the oxygen concentration in the river falls below the saturation concentration. Consequently, oxygen is transferred from the atmosphere to the river. The rate of this transfer at position z, written TDO(z) (in mg of oxygen transferred per day and per litre of river), is expressed as follows: TDO(z) = k2(DOsat - DO(z)), where DOsat is the concentration of dissolved oxygen saturation (taken equal to 8 mg/l in this problem) and DO(z) is the dissolved oxygen concentration at position z in the river.
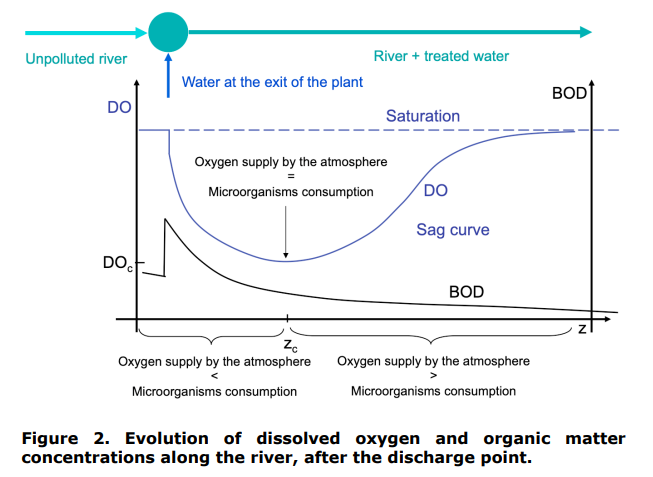
As one moves away from the point of discharge, the concentration of dissolved oxygen in the river decreases. So the rate of oxygen transfer from the atmosphere to the river increases. In addition, as one moves away from the discharge point, the concentration of organic matter in the river decreases, therefore the rate of oxygen consumption decreases too. Consequently, there is a location in the river where the rate of oxygen transfer from the atmosphere to the river is equal to the rate of consumption of oxygen by the microorganisms. This point corresponds to a minimum in the DO(z) curve. Beyond this point, the replenishment of oxygen from the atmosphere takes over the consumption of oxygen by the microorganisms. Consequently, the concentration of dissolved oxygen in the river gradually rises to the saturation concentration.
Typical values of the coefficient k1 are given in Table 1. These are averages over a large number of organic compounds. Molecules such as sugars have a large k1, while complex molecules like phenols have a small k1. The influence of the temperature on k1 can be expressed as follows:
k1 (T ) = k1 (T = 20C)T 20 , with = 1.047
It is a relation established empirically.
| k1 (day-1 ), at 20 C | BOD (mg/l) | |
| Tap water | 0 1 | |
| Surface water | 0.1 0.23 | 1 30 |
| Untreated UWW | 0.35 0.4 | 150 250 |
| Treated UWW | 0.12 0.23 | 10 40 |
Typical values of the coefficient k2 are given in Table 2. k2 is influenced by the intensity of the turbulence, the ratio of the surface area to the volume of the river and the temperature. The influence of the temperature on k2 can be expressed as follows:
k2(T)= k2(T= 20C) T-20 , with = 1.016
It is a relation established empirically.
| k2 (day-1 ), at 20 C | |
| Lake | 0.1 0.23 |
| Small river with small flow rate | 0.23 0.35 |
| Common river with usual flow rate | 0.35 0.46 |
| Large river with huge flow rate | 0.46 1.15 |
| Rapids and waterfalls | > 1.15 |
Question 1.
By considering a river element between the positions z and z + dz, please establish, by writing out two mass balances (see Figures 3 and 4), the differential equations describing the evolution of the concentrations of organic matter and dissolved oxygen along the river. We assume that the river can be described by a plug flow reactor (PFTR). We write u (m/s) the average velocity of water in the river. The area of the section of the river perpendicular to the flow is written (m2 ).
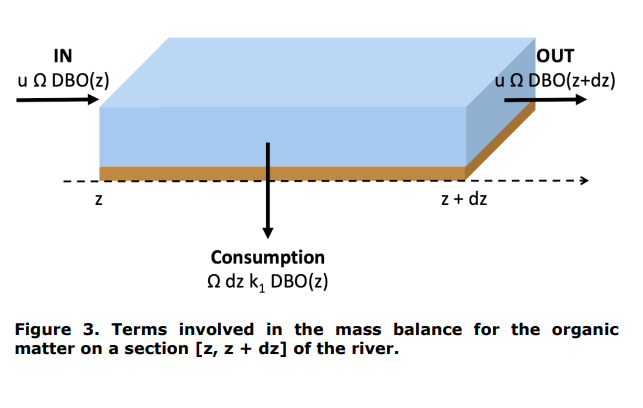
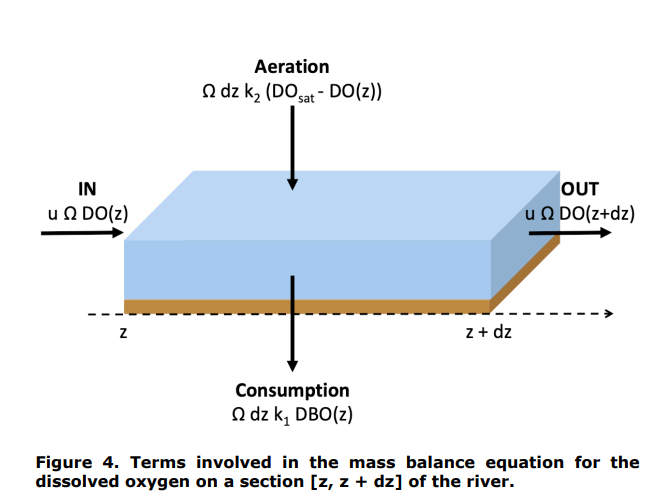
Question 2.
Solve the differential equations obtained in question 1. To simplify the notations, please write: DO(z = 0) = DO0 and DBO(z = 0) = DBO0.
Question 3.
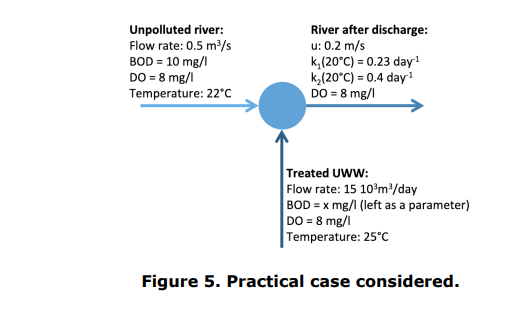
According to the data presented in Figure 5, calculate the maximum value that the BOD of the treated UWW (x) can take in order to prevent the concentration of dissolved oxygen in the river falling below 3 mg/l. It is a critical concentration because, below this concentration, the fish present in the river are no longer able to breathe correctly.
For this value maximal value of the BOD of the treated UWW, how far from the discharge location is the minimum dissolved oxygen concentration in the river observed?
Introduction. Figure 1. Discharge of a treated urban wastewater in a river. Figure 2. Evolution of dissolved oxygen and organic matter concentrations along the river, after the discharge point. Figure 3. Terms involved in the mass balance for the organic matter on a section [z,z+dz] of the river. dissolved oxygen on a section [z,z+dz] of the river. Figure 5. Practical case consideredStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


