Question
Incineration (also called waste-to-energy) is a combustion process where oxygen is used at high temperature to liberate the energy in the waste. A refuse-derived fuel
Incineration (also called waste-to-energy) is a combustion process where oxygen is used at high temperature to liberate the energy in the waste. A refuse-derived fuel (RDF) compromises of 60% mixed paper, 30% mixed plastic, and 10% textiles. Assume it is completely dried before combustion. Determine the volume of air (in L) at 20 degrees Celsius, 1 atmosphere pressure that is required to combust 1kg of the RDF.
Use the values from table to determine the molecules of C, H, S, and O in the waste, the molecular weights of C,H,S and O are, 12,1,32,16g/mol
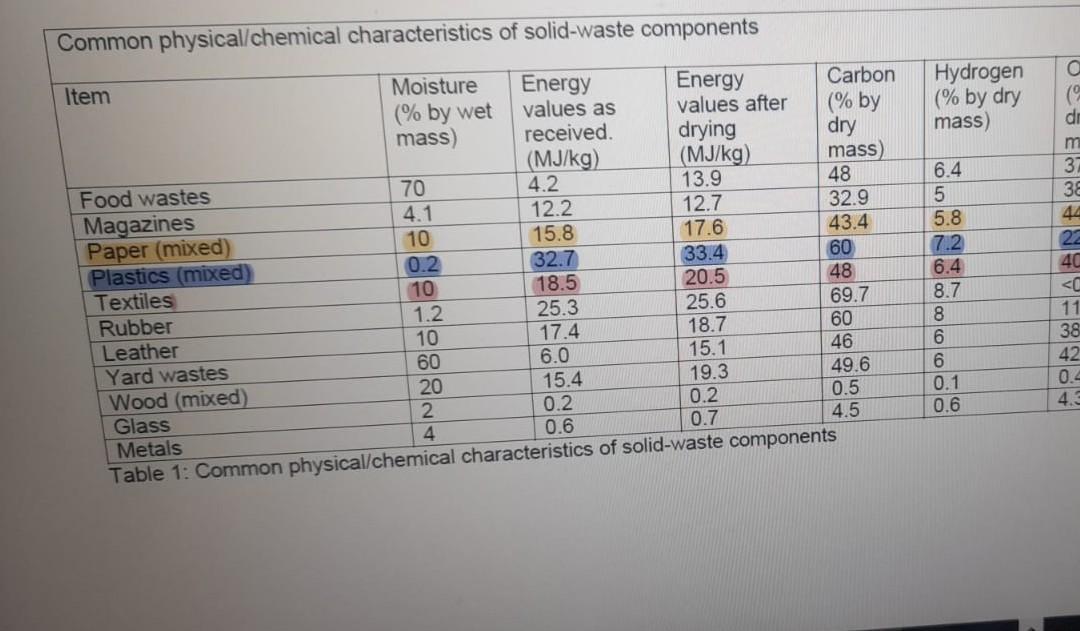
Common physical/chemical characteristics of solid-waste components Energy values after Item Food wastes Magazines Paper (mixed) Plastics (mixed) Textiles Rubber Leather Moisture (% by wet mass) 70 4.1 10 0.2 10 1.2 10 60 Energy values as received. (MJ/kg) 20 2 4 4.2 12.2 15.8 32.7 18.5 25.3 17.4 6.0 15.4 0.2 0.6 drying (MJ/kg) 13.9 12.7 17.6 33.4 20.5 25.6 18.7 15.1 19.3 0.2 0.7 Carbon (% by dry mass) 48 32.9 43.4 60 48 69.7 60 46 49.6 0.5 4.5. Yard wastes Wood (mixed) Glass Metals Table 1: Common physical/chemical characteristics of solid-waste components Hydrogen (% by dry mass) 6.4 65579 5.8 7.2 6.4 8.7 8 0.1 0.6 a APACHABRA WW3 O ( dr m 38 44 22 40 < 11 38 42 0.4 4.3
Step by Step Solution
3.51 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Solution Step 1 a First we will find the volume of air required at 20C and 1 atmosphere pressure Her...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


