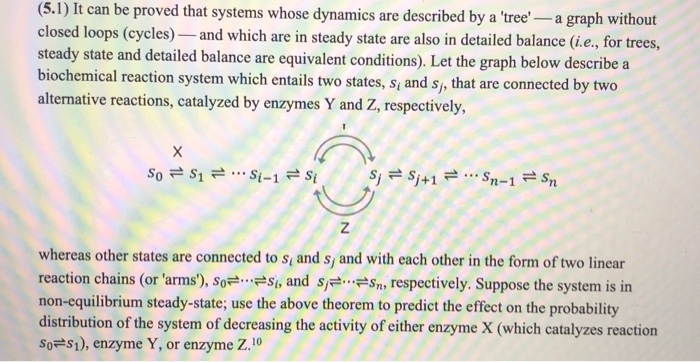
(5.1) It can be proved that systems whose dynamics are described by a 'tree' -- a graph without closed loops (cycles) and which are in steady state are also in detailed balance (i.e., for trees, steady state and detailed balance are equivalent conditions). Let the graph below describe a biochemical reaction system which entails two states, s, and s, that are connected by two alternative reactions, catalyzed by enzymes Y and Z, respectively, So S1 = ..Si-1 si S; S;+1 Sn-1 Sn whereas other states are connected to s and s, and with each other in the form of two linear reaction chains (or 'arms'), SO=...=si, and s=s=sm, respectively. Suppose the system is in non-equilibrium steady-state; use the above theorem to predict the effect on the probability distribution of the system of decreasing the activity of either enzyme X (which catalyzes reaction So=S1), enzyme Y, or enzyme Z.10 consequences. Thus, the cell must maintain a nonequilibrium steady state: i.e., export entropy into the environment at the rate of its production - the cell must be open to its environment with which it must exchange energy (also in the form of matter). The problem now is this. How to maintain stable steady state conditions while being openly embedded into a constantly changing environment? We may imagine a chain of reactions as a chain of Bernoulli processes, s, 5+1, with shared nodes. The nodes, s, (chemical states), are occupied by a generalized molecule X which occupies each state with some probability p,t)We thus can speak of the flux of probability mass in the chain since, in steady state, the total mass (like total probability) within the chain is! constant. As we have seen, metabolic enzymes create reaction topologies with closed loops, "futile" reaction cycles. The futile cycle is the topological motif of metabolism. So = $1 = ...Si-1 =si S; =S;+1 = -Sn-1 = Sn Because of coupling to the ATP/ADP system, both reactions of a futile cycle are exergonic in opposite directions. The reaction system is not in equilibrium, therefore. Yet, as a consequence of graph topology and steady state condition, all reactions other than futile cycle reactions are in equilibrium - even when the system as a whole is not. The table below lists the free energy changes of all glycolytic reaction under standard and in the conditions (muscle cells). Reaction Enzyme AG" (kJ. mol!) AG (kJ. mol-)) HK -20.9 -272 PGI +2.2 -1.4 PFK - 17.2 259 Aldolase +22.8 -5.9 TIM +7.9 Negative 6 +7 GAPDH + PGK -10.7 -1.1 PGM -0.6 Enolase -3.2 -2.4 10 -210 - 13.9 "Calculated from data in Newsholme, E.A. and Start, C. Regulation in Metabolism 97. Wiley (1973). +4.7 As predicted by our analysis, reactions that are not part of futile cycles are close to equilibrium Le AG values are close to zero - whereas futile reaction cycle reactions are not. Deviations reached from any other node by a string of (directed) edges. We model molecular dynamics as probability flow across strongly connected graphs. A strongly connected graph Let p; (c) be the probability that the system occupies state j at time t. The time evolution of the probability distribution across the graph is given by the master equation', which accounts for the flux of probability mass into and out of each state : dp/(t)- wyp,(1) - wp.(t). dt where w is the rate constant for the flow of probability mass from microscopic state into state 1. The first sum on the right is the total flow of probability per unit time (flux') mass into state i at time t; the second sum is the total flux out of i. The master equation, thus, has the same form as rate equations for elementary chemical reactions (law of mass action'), with probability taking the place of concentration. With wu = - w we may rewrite the master equation more simply: dp () - Swyp,(t). The master equation entails the 'Markov assumption'--that the future evolution of probabilities depends entirely on the present probability distribution pol. and not the past. It can be proved (but we won't do this here that the reverse is true, too: the Markov assumption implies the master equation. Thus, the master equation and Markov assumption are logically equivalent The mass total of probability is conserved, P(1) - lile.. (2 2,0) - Thus, by summing both sides of the master equation over all 1, we find that 27,60 = 3 (E-0) = 0. (5.1) It can be proved that systems whose dynamics are described by a 'tree' -- a graph without closed loops (cycles) and which are in steady state are also in detailed balance (i.e., for trees, steady state and detailed balance are equivalent conditions). Let the graph below describe a biochemical reaction system which entails two states, s, and s, that are connected by two alternative reactions, catalyzed by enzymes Y and Z, respectively, So S1 = ..Si-1 si S; S;+1 Sn-1 Sn whereas other states are connected to s and s, and with each other in the form of two linear reaction chains (or 'arms'), SO=...=si, and s=s=sm, respectively. Suppose the system is in non-equilibrium steady-state; use the above theorem to predict the effect on the probability distribution of the system of decreasing the activity of either enzyme X (which catalyzes reaction So=S1), enzyme Y, or enzyme Z.10 consequences. Thus, the cell must maintain a nonequilibrium steady state: i.e., export entropy into the environment at the rate of its production - the cell must be open to its environment with which it must exchange energy (also in the form of matter). The problem now is this. How to maintain stable steady state conditions while being openly embedded into a constantly changing environment? We may imagine a chain of reactions as a chain of Bernoulli processes, s, 5+1, with shared nodes. The nodes, s, (chemical states), are occupied by a generalized molecule X which occupies each state with some probability p,t)We thus can speak of the flux of probability mass in the chain since, in steady state, the total mass (like total probability) within the chain is! constant. As we have seen, metabolic enzymes create reaction topologies with closed loops, "futile" reaction cycles. The futile cycle is the topological motif of metabolism. So = $1 = ...Si-1 =si S; =S;+1 = -Sn-1 = Sn Because of coupling to the ATP/ADP system, both reactions of a futile cycle are exergonic in opposite directions. The reaction system is not in equilibrium, therefore. Yet, as a consequence of graph topology and steady state condition, all reactions other than futile cycle reactions are in equilibrium - even when the system as a whole is not. The table below lists the free energy changes of all glycolytic reaction under standard and in the conditions (muscle cells). Reaction Enzyme AG" (kJ. mol!) AG (kJ. mol-)) HK -20.9 -272 PGI +2.2 -1.4 PFK - 17.2 259 Aldolase +22.8 -5.9 TIM +7.9 Negative 6 +7 GAPDH + PGK -10.7 -1.1 PGM -0.6 Enolase -3.2 -2.4 10 -210 - 13.9 "Calculated from data in Newsholme, E.A. and Start, C. Regulation in Metabolism 97. Wiley (1973). +4.7 As predicted by our analysis, reactions that are not part of futile cycles are close to equilibrium Le AG values are close to zero - whereas futile reaction cycle reactions are not. Deviations reached from any other node by a string of (directed) edges. We model molecular dynamics as probability flow across strongly connected graphs. A strongly connected graph Let p; (c) be the probability that the system occupies state j at time t. The time evolution of the probability distribution across the graph is given by the master equation', which accounts for the flux of probability mass into and out of each state : dp/(t)- wyp,(1) - wp.(t). dt where w is the rate constant for the flow of probability mass from microscopic state into state 1. The first sum on the right is the total flow of probability per unit time (flux') mass into state i at time t; the second sum is the total flux out of i. The master equation, thus, has the same form as rate equations for elementary chemical reactions (law of mass action'), with probability taking the place of concentration. With wu = - w we may rewrite the master equation more simply: dp () - Swyp,(t). The master equation entails the 'Markov assumption'--that the future evolution of probabilities depends entirely on the present probability distribution pol. and not the past. It can be proved (but we won't do this here that the reverse is true, too: the Markov assumption implies the master equation. Thus, the master equation and Markov assumption are logically equivalent The mass total of probability is conserved, P(1) - lile.. (2 2,0) - Thus, by summing both sides of the master equation over all 1, we find that 27,60 = 3 (E-0) = 0