Question
INFO:: Used 3 ml styreNE 0.40 ml initiator tert butyl obtained 0.8660 g polystyrene polymerized 1.5512 g styrene QUESTIONS : 1. A feature of polymers

INFO:: Used 3 ml styreNE
0.40 ml initiator tert butyl
obtained 0.8660 g polystyrene
polymerized 1.5512 g styrene
QUESTIONS:
1. A feature of polymers compared to “normal” molecules, which have fixed molecular weights, is that the same polymer can have a range of molecular weights depending on the conditions under which it was made. For the reaction we did, assume each initiator molecule starts one polystyrene chain and each chain is the same length, calculate the number of styrene molecules per chain and thus the weight of a chain of YOUR polymer.
2. Suggest a reason why methanol (the solvent used in this lab) is a better solvent for isolating polystyrene as a powder than acetone.
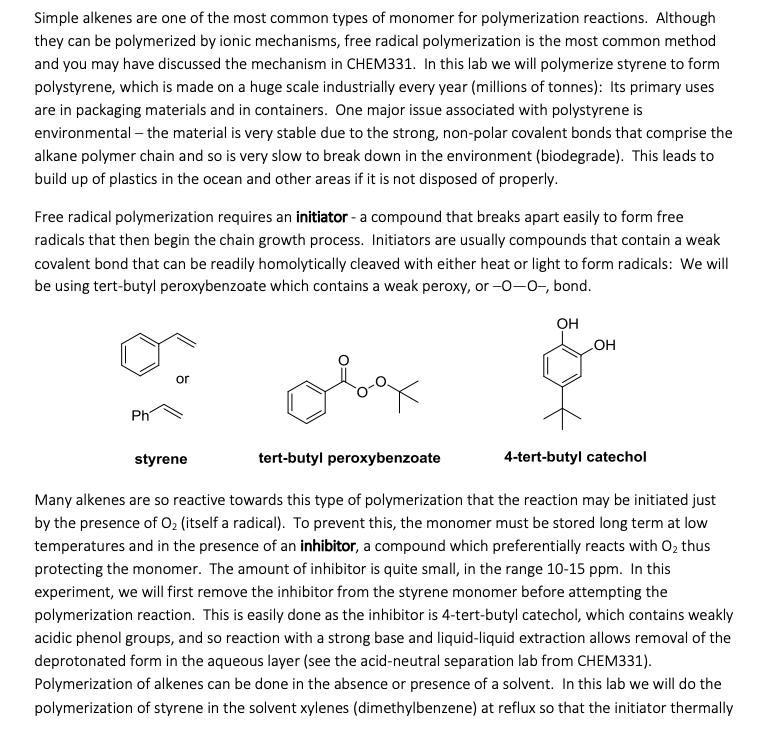
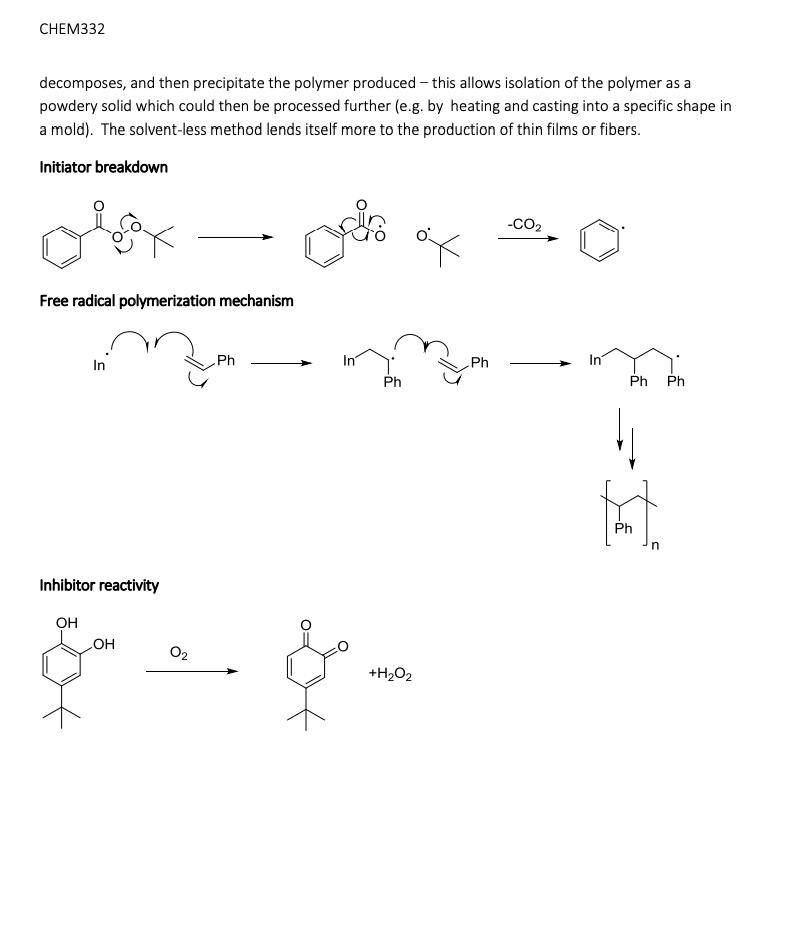
Simple alkenes are one of the most common types of monomer for polymerization reactions. Although they can be polymerized by ionic mechanisms, free radical polymerization is the most common method and you may have discussed the mechanism in CHEM331. In this lab we will polymerize styrene to form polystyrene, which is made on a huge scale industrially every year (millions of tonnes): Its primary uses are in packaging materials and in containers. One major issue associated with polystyrene is environmental - the material is very stable due to the strong, non-polar covalent bonds that comprise the alkane polymer chain and so is very slow to break down in the environment (biodegrade). This leads to build up of plastics in the ocean and other areas if it is not disposed of properly. Free radical polymerization requires an initiator - a compound that breaks apart easily to form free radicals that then begin the chain growth process. Initiators are usually compounds that contain a weak covalent bond that can be readily homolytically cleaved with either heat or light to form radicals: We will be using tert-butyl peroxybenzoate which contains a weak peroxy, or -0-0-, bond. OH Ph or tert-butyl peroxybenzoate OH 4-tert-butyl catechol styrene Many alkenes are so reactive towards this type of polymerization that the reaction may be initiated just by the presence of O (itself a radical). To prevent this, the monomer must be stored long term at low temperatures and in the presence of an inhibitor, a compound which preferentially reacts with O thus protecting the monomer. The amount of inhibitor is quite small, in the range 10-15 ppm. In this experiment, we will first remove the inhibitor from the styrene monomer before attempting the polymerization reaction. This is easily done as the inhibitor is 4-tert-butyl catechol, which contains weakly acidic phenol groups, and so reaction with a strong base and liquid-liquid extraction allows removal of the deprotonated form in the aqueous layer (see the acid-neutral separation lab from CHEM331). Polymerization of alkenes can be done in the absence or presence of a solvent. In this lab we will do the polymerization of styrene in the solvent xylenes (dimethylbenzene) at reflux so that the initiator thermally
Step by Step Solution
3.35 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Detailed Explanation The experiment involves the use of tertbutyl peroxybenzoate as an initiator for free radical polymerization The process also includes the removal of the inhibitor 4tertbutyl catec...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


