Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Instructions. Carefully review the Constant-Volume Standard Addition material (pages 7-13) of the CHEM 146 Lab 2 Manual given its central importance (especially the fully worked
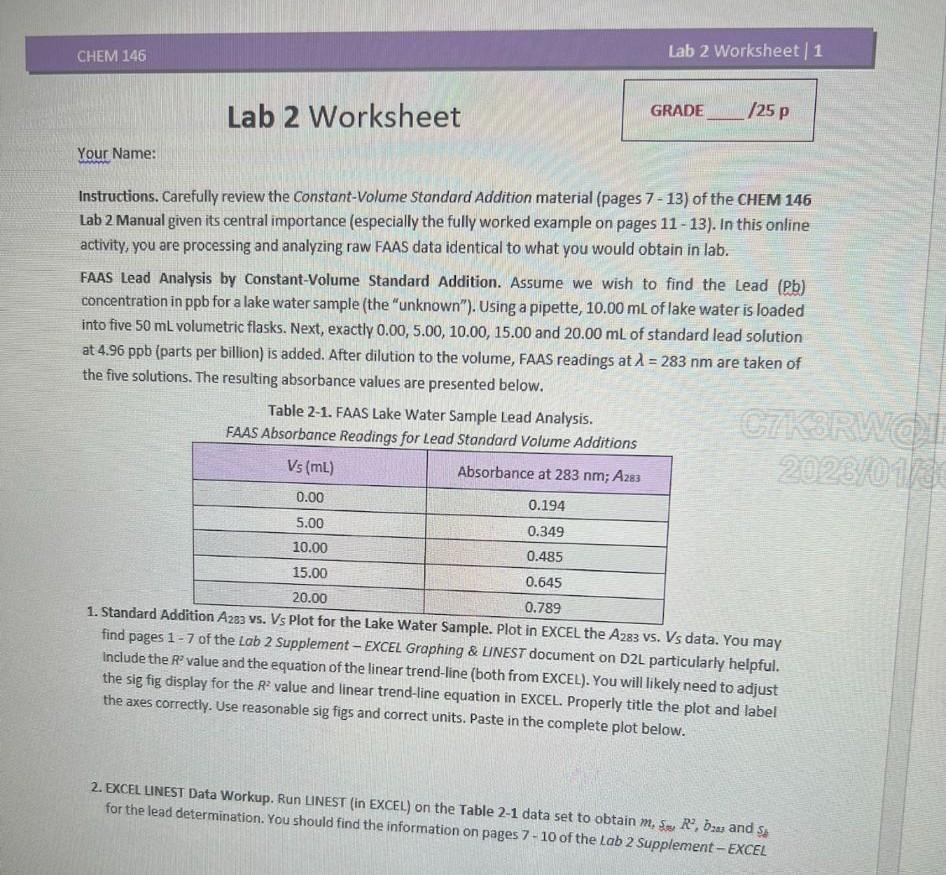
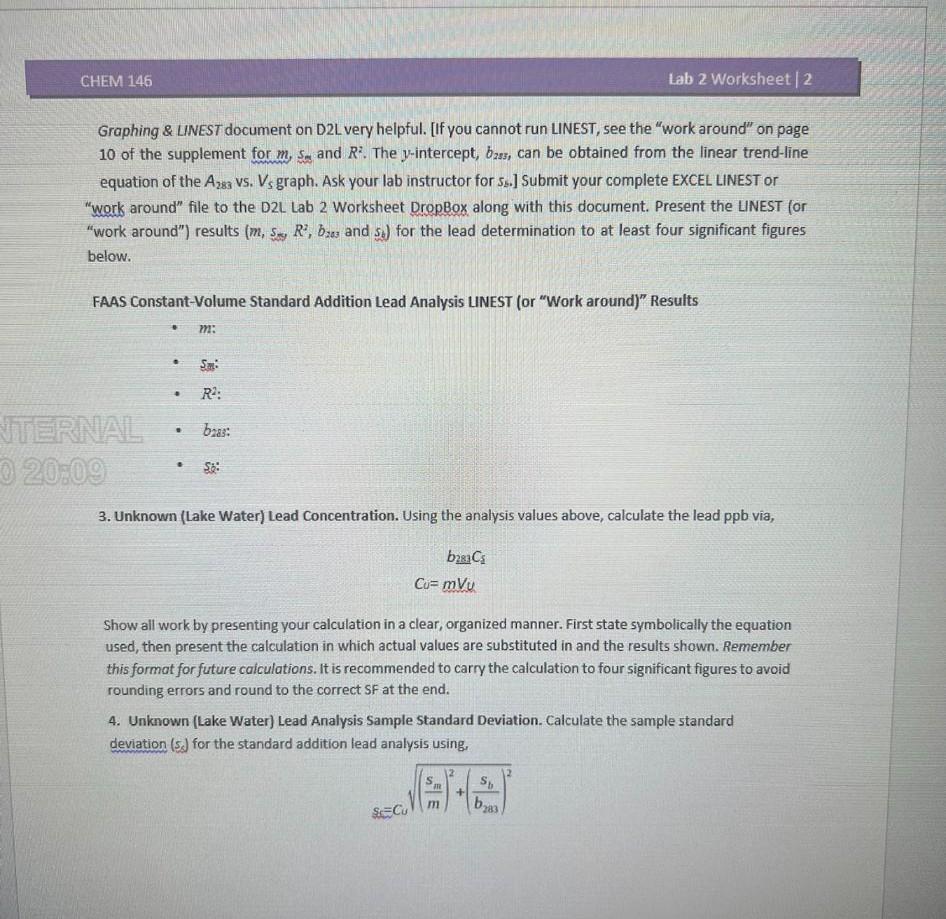

Instructions. Carefully review the Constant-Volume Standard Addition material (pages 7-13) of the CHEM 146 Lab 2 Manual given its central importance (especially the fully worked example on pages 1113 ). In this online activity, you are processing and analyzing raw FAAS data identical to what you would obtain in lab. FAAS Lead Analysis by Constant-Volume Standard Addition. Assume we wish to find the Lead ( Pb) concentration in ppb for a lake water sample (the "unknown"). Using a pipette, 10.00mL of lake water is loaded into five 50mL velumetric flasks. Next, exactly 0.00,5.00,10.00,15.00 and 20.00mL of standard lead solution at 4.96ppb (parts per billion) is added. After dilution to the volume, FAAS readings at =283nm are taken of the five solutions. The resulting absorbance values are presented below. Table 2-1. FAAS Lake Water Sample Lead Analysis. FAAS Absorbance Readings for Lead Standard Vnlumo Additinm 1. Standard Adc find pages 1 - 7 of the Lab 2 Supplement - EXCEL Grapher Sample. Plot in EXCEL the A283 vs. Vs data. You may Include the R2 value and the equation of the ling E ERaphing \& LINEST document on D2L particularly helpful. the sig fig display for the R2 value and linear the axes correctly. Use reasonable sig figs and corend-line equation in EXCEL. Properly title the plot and label axigh figs and correct units. Paste in the complete plot below. 2. EXCEL LINEST Data Workup. Run LINEST (in EXCEL) on the Table 21 data set to obtain m,ss,R2,b2as and s6 for the lead determination. You should find the information on pages 7 - 10 of the lab 2 Supplement - EXCEL Graphing \& LINEST document on D2L very helpful. [If you cannot run LINEST, see the "work around" on page 10 of the supplement for m,s5 and R2. The y-intercept, b2ss, can be obtained from the linear trend-line equation of the A283 Vs. VS graph. Ask your lab instructor for Ss.] Submit your complete EXCEL LINEST or "work around" file to the D2L Lab 2 Worksheet DropBox along with this document. Present the LINEST for "work around") results (m,ss,s,R2,b2as and sj) for the lead determination to at least four significant figures below. FAAS Constant-Volume Standard Addition Lead Analysis LINEST (or "Work around)" Results - m: - ss: - R2 : - bras: - Si: 3. Unknown (Lake Water) Lead Concentration. Using the analysis values above, calculate the lead ppb via, Cu=mVu Show all work by presenting your calculation in a clear, organized manner. First state symbolically the equation used, then present the calculation in which actual values are substituted in and the results shown. Remember this format for future caiculations. It is recommended to carry the calculation to four significant figures to avoid rounding errors and round to the correct SF at the end. 4. Unknown (Lake Water) Lead Analysis Sample Standard Deviation. Calculate the sample standard deviation (ss) for the standard addition lead analysis using, sc=Cu(msm)2+(b233sb)2 Show all work by presenting your calculations in a clear, organized manner. Remember to first state symbolically the equation, then present the calculation with actual values substituted in and the result shown. It is recommended to carry the calculation to four significant figures to avoid rounding errors and only round to the correct SF at the end. 5. Unknown (Lake Water) Lead Analysis Mean and Sample Standard Deviation. Write the lead analysis results in the form Cutss for the lake water sample in the space below. (For your reference, the EPA action threshold for lead in public drinking water systems is 15ppb, or 15 g/L.) Instructions. Carefully review the Constant-Volume Standard Addition material (pages 7-13) of the CHEM 146 Lab 2 Manual given its central importance (especially the fully worked example on pages 1113 ). In this online activity, you are processing and analyzing raw FAAS data identical to what you would obtain in lab. FAAS Lead Analysis by Constant-Volume Standard Addition. Assume we wish to find the Lead ( Pb) concentration in ppb for a lake water sample (the "unknown"). Using a pipette, 10.00mL of lake water is loaded into five 50mL velumetric flasks. Next, exactly 0.00,5.00,10.00,15.00 and 20.00mL of standard lead solution at 4.96ppb (parts per billion) is added. After dilution to the volume, FAAS readings at =283nm are taken of the five solutions. The resulting absorbance values are presented below. Table 2-1. FAAS Lake Water Sample Lead Analysis. FAAS Absorbance Readings for Lead Standard Vnlumo Additinm 1. Standard Adc find pages 1 - 7 of the Lab 2 Supplement - EXCEL Grapher Sample. Plot in EXCEL the A283 vs. Vs data. You may Include the R2 value and the equation of the ling E ERaphing \& LINEST document on D2L particularly helpful. the sig fig display for the R2 value and linear the axes correctly. Use reasonable sig figs and corend-line equation in EXCEL. Properly title the plot and label axigh figs and correct units. Paste in the complete plot below. 2. EXCEL LINEST Data Workup. Run LINEST (in EXCEL) on the Table 21 data set to obtain m,ss,R2,b2as and s6 for the lead determination. You should find the information on pages 7 - 10 of the lab 2 Supplement - EXCEL Graphing \& LINEST document on D2L very helpful. [If you cannot run LINEST, see the "work around" on page 10 of the supplement for m,s5 and R2. The y-intercept, b2ss, can be obtained from the linear trend-line equation of the A283 Vs. VS graph. Ask your lab instructor for Ss.] Submit your complete EXCEL LINEST or "work around" file to the D2L Lab 2 Worksheet DropBox along with this document. Present the LINEST for "work around") results (m,ss,s,R2,b2as and sj) for the lead determination to at least four significant figures below. FAAS Constant-Volume Standard Addition Lead Analysis LINEST (or "Work around)" Results - m: - ss: - R2 : - bras: - Si: 3. Unknown (Lake Water) Lead Concentration. Using the analysis values above, calculate the lead ppb via, Cu=mVu Show all work by presenting your calculation in a clear, organized manner. First state symbolically the equation used, then present the calculation in which actual values are substituted in and the results shown. Remember this format for future caiculations. It is recommended to carry the calculation to four significant figures to avoid rounding errors and round to the correct SF at the end. 4. Unknown (Lake Water) Lead Analysis Sample Standard Deviation. Calculate the sample standard deviation (ss) for the standard addition lead analysis using, sc=Cu(msm)2+(b233sb)2 Show all work by presenting your calculations in a clear, organized manner. Remember to first state symbolically the equation, then present the calculation with actual values substituted in and the result shown. It is recommended to carry the calculation to four significant figures to avoid rounding errors and only round to the correct SF at the end. 5. Unknown (Lake Water) Lead Analysis Mean and Sample Standard Deviation. Write the lead analysis results in the form Cutss for the lake water sample in the space below. (For your reference, the EPA action threshold for lead in public drinking water systems is 15ppb, or 15 g/L.)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


