Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Instructions: Plot graph one: [A]/M (y axis) versus time/hours (x axis). Be sure to correctly label your axis with the appropriate unit. Scale your graph

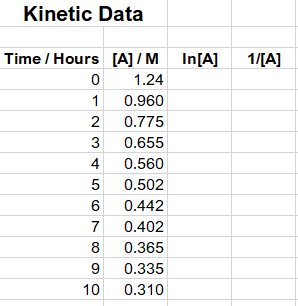
Instructions:
- Plot graph one: [A]/M (y axis) versus time/hours (x axis). Be sure to correctly label your axis with the appropriate unit. Scale your graph appropriately so takes up the enter canvas. Decide on the type of trendline that best fits the data points.
- Plot graph two: ln[A] versus time/ hours. Label and scale your graph properly. Decide on the type of trendline that best fits the data points.
- Plot graph three; 1/[A] versus time. Label and scale your graph properly. Again decide on the type of trendline that best fits the data points.
- Only the graph that appears linear should have a linear trendline along with the equation for the line and the correlation coefficient. The other two graphs should be curves with no equations or coefficients.
- From your plots determine if the reaction is first, second or third order.
- At what time will the concentration of A be 0.380 M? Show all your work.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


