Answered step by step
Verified Expert Solution
Question
1 Approved Answer
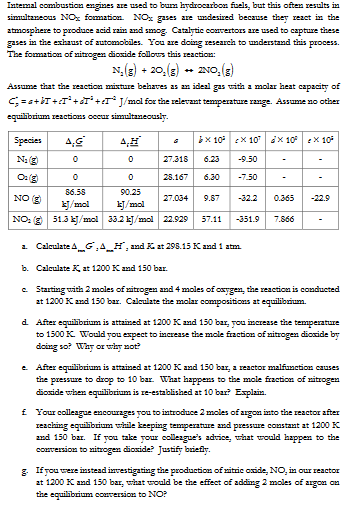
Intemal combustion engines are used to bum hydrocarbon fuels, but this often results in simultaneous N * O _ { x } formation. N *
Intemal combustion engines are used to bum hydrocarbon fuels, but this often results in simultaneous NOx formation. NOx gases are undesired because they react in the atmosphere to produce acid rain and smog. Catalytic convertors are used to capture these gases in the exhaust of automobiles. You are doing research to understand this process. The formation of nitrogen dioxide follous this reaction:
N gO g leftrightarrow Og
Assume that the reaction mixture behaves as an ideal gas with a molar heat capacity of C g prime a bT epsilon T d T epsilon T J mol for the relevant temperature range. Assume no other equilibrium reactions occur simultaneously.
Species
Deltat subseteq
HV
angle
X
mathcalNmathfrakg
NO
kJmol
kJmol
NO: g
kJmol
kJmol
i
Calculate DeltamG Delta min H and K at K and atm.
b Calculate K at K and bar.
c Starting with moles of nitrogen and moles of oxygen, the reaction is conducted at K and bar. Calculate the molar compositions at equilibrium
d After equilibrium is attained at K and bar, you increase the temperature to K Would you expect to increase the mole fraction of nitrogen dioxide by doing so Why or why not?
e After equilibrium is attained at K and bar, a reactor malfunction causes the pressure to drop to bar. What happens to the mole fraction of nitrogen dioxide when equilibrium is zeestablished at bar? Explain
f Your colleague encourages you to introduce moles of argon into the reactor after reaching equilibrium while keeping temperature and pressure constant at K and bar. If you take your colleague's advice, what would happen to the conversion to nitrogen dioxide? Justify briefly.
g If you were instead investigating the production of nitric oxide, NO in our reactor at K and bar, what would be the effect of adding moles of argon on the equilibrium conversion to NO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


