Answered step by step
Verified Expert Solution
Question
1 Approved Answer
into a binary mixture consisting of components A (18 g/mol) and B (46 g/mol). The molar density property of It is expressed by the equation
into a binary mixture consisting of components A (18 g/mol) and B (46 g/mol).
The molar density property of
It is expressed by the equation below.
Here, = 5x10^3
, = 1x10^2 and = 2x10^-2, having the unit mol/cm3.
Construct the graph of 1 based on the above information. also Partial molar components of A (20% by weight) and B (80% by weight) in solution
Calculate their volume.
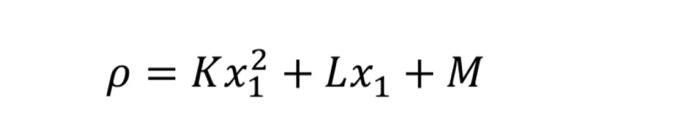
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


