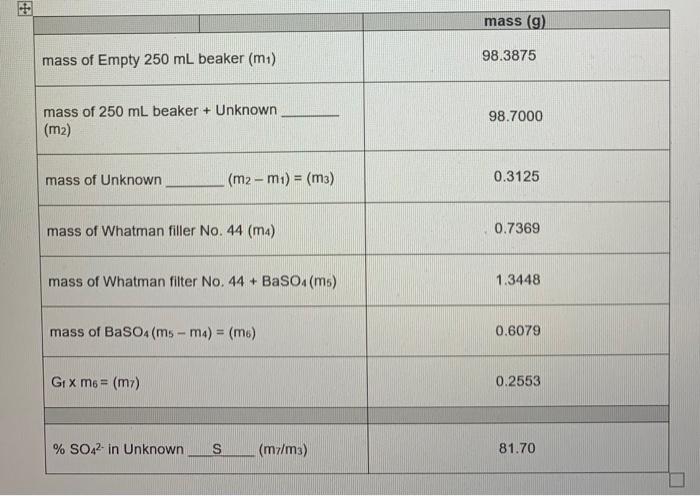
Introduction: Gravimetric analysis is an analytical method used to determine the amount of a specific analyte present in an unknown solid/solution. Gravimetric analysis is based upon the selective precipitation of a specific analyte using various reagents. The precipitate is collected, washed and weighed. Based on the type of precipitate formed and its mass, the amount of analyte may be calculated using a "gravimetric factor", which represents the mass ratio of analyte to the precipitate. Mass Analyte = Mass Precipitate x Gravimetric Factor (GS) Purpose: In this experiment, the student will determine the percentage sulphate present in an unknown solid sample. Reagents: Deionized water (DI) 6 M HCI (Hydrochloric acid) 0.1 M BaCl2 (Barium Chloride) Equipment: [1] 250 ml beaker (1) 50.00 ml. Volumetric pipet [1] 25.00 ml Volumetric pipet [1]250 ml. Erlenmeyer flask (Filtration) Pasteur pipet ( 1 ) stirring rod [1] Air Cadet Vacuum/pressure station with rubber tubing [1] Hot plate [1] Glass funnel [1] Whatman 44 Ashless filter paper (110 mm diameter) Analytical Balance Procedure: 1. Measure and record the mass of a clean, dry 250 ml beaker using the analytical balance to the nearest 0.0001 g. Add between 0.30 g and 0.35 g of your unknown sample to the 250 ml beaker and record the mass of the beaker plus sample to the nearest 0.0001 g. 2. Using a 50.00 mL volumetric pipet, add 50 mL of DI to the sample in the beaker. 3. Next, add 20 drops of 6 M HCI (using Pasteur pipet). Stir the contents of the beaker until the sample has entirely dissolved. Leave the stirring rod in the beaker. 4. Measure 25 mL of 0.1 M BaCl2 solution in a 25.00 ml. Volumetric pipet. 5. Heat the solution containing the sample in the 250 ml beaker until it is nearly boiling. Turn the hot plate off. 6. Slowly pour small portions of the 0.1 M BaCl2 solution into the 250 ml beaker containing the sample. This step should take AT LEAST 3 minutes, otherwise the BaCl2 is being added too rapidly. It is important to stir the contents of the beaker as the BaCl2 solution is being added. You should observe the formation of the white Baso, precipitate. Rinse any precipitate that remains on the stirring rod into the solution with a small amount of Dl and allow the precipitate to settle in the beaker for about 20 minutes. 7. While the precipitate settles, obtain a piece of Whatman 44 ashless filter paper (110 mm diameter) and weigh it on the analytical balance. Record the mass to the nearest +0.0001 g. Fold it into quarters. Open the folded paper into a cone and place it into a glass funnel and wet the filter paper with DI so that it sticks' to the funnel. Place a 250 ml Filtration Erlenmeyer flask (attached to Vacuum/pressure station with rubber tubing) under the funnel to collect the filtrate. 8. After 20 minutes has passed, slowly pour the mixture containing the Basos precipitate down your stirring rod into the funnel. Be careful that the level of the liquid in the funnel is never more than three-fourths of the way to the top of the filter paper. When the transfer is complete use your wash bottle (filled with DI) to rinse the residual precipitate from the beaker and the stirring rod into the funnel. a 9. After all the liquid has drained from the funnel very carefully press the top edges of the filter paper together and remove it from the funnel. Place the filter paper in the oven @ 100 C for 20 minutes. 10. Allow to air dry overnight. 11. The next day, record the mass of the filter paper and its contents to the nearest +0.0001 g on the same analytical balance. mass (g) mass of Empty 250 ml beaker (m1) 98.3875 mass of 250 mL beaker + Unknown (m2) 98.7000 mass of Unknown (m2-mi) = (m3) 0.3125 mass of Whatman filler No. 44 (m) 0.7369 mass of Whatman filter No.44 + BaSO4(ms) 1.3448 mass of BaSO4(ms-ma) = (m6) 0.6079 Gtx m6 = (m) 0.2553 % SO42- in Unknown S (mz/m3) 81.70 U Introduction: Gravimetric analysis is an analytical method used to determine the amount of a specific analyte present in an unknown solid/solution. Gravimetric analysis is based upon the selective precipitation of a specific analyte using various reagents. The precipitate is collected, washed and weighed. Based on the type of precipitate formed and its mass, the amount of analyte may be calculated using a "gravimetric factor", which represents the mass ratio of analyte to the precipitate. Mass Analyte = Mass Precipitate x Gravimetric Factor (GS) Purpose: In this experiment, the student will determine the percentage sulphate present in an unknown solid sample. Reagents: Deionized water (DI) 6 M HCI (Hydrochloric acid) 0.1 M BaCl2 (Barium Chloride) Equipment: [1] 250 ml beaker (1) 50.00 ml. Volumetric pipet [1] 25.00 ml Volumetric pipet [1]250 ml. Erlenmeyer flask (Filtration) Pasteur pipet ( 1 ) stirring rod [1] Air Cadet Vacuum/pressure station with rubber tubing [1] Hot plate [1] Glass funnel [1] Whatman 44 Ashless filter paper (110 mm diameter) Analytical Balance Procedure: 1. Measure and record the mass of a clean, dry 250 ml beaker using the analytical balance to the nearest 0.0001 g. Add between 0.30 g and 0.35 g of your unknown sample to the 250 ml beaker and record the mass of the beaker plus sample to the nearest 0.0001 g. 2. Using a 50.00 mL volumetric pipet, add 50 mL of DI to the sample in the beaker. 3. Next, add 20 drops of 6 M HCI (using Pasteur pipet). Stir the contents of the beaker until the sample has entirely dissolved. Leave the stirring rod in the beaker. 4. Measure 25 mL of 0.1 M BaCl2 solution in a 25.00 ml. Volumetric pipet. 5. Heat the solution containing the sample in the 250 ml beaker until it is nearly boiling. Turn the hot plate off. 6. Slowly pour small portions of the 0.1 M BaCl2 solution into the 250 ml beaker containing the sample. This step should take AT LEAST 3 minutes, otherwise the BaCl2 is being added too rapidly. It is important to stir the contents of the beaker as the BaCl2 solution is being added. You should observe the formation of the white Baso, precipitate. Rinse any precipitate that remains on the stirring rod into the solution with a small amount of Dl and allow the precipitate to settle in the beaker for about 20 minutes. 7. While the precipitate settles, obtain a piece of Whatman 44 ashless filter paper (110 mm diameter) and weigh it on the analytical balance. Record the mass to the nearest +0.0001 g. Fold it into quarters. Open the folded paper into a cone and place it into a glass funnel and wet the filter paper with DI so that it sticks' to the funnel. Place a 250 ml Filtration Erlenmeyer flask (attached to Vacuum/pressure station with rubber tubing) under the funnel to collect the filtrate. 8. After 20 minutes has passed, slowly pour the mixture containing the Basos precipitate down your stirring rod into the funnel. Be careful that the level of the liquid in the funnel is never more than three-fourths of the way to the top of the filter paper. When the transfer is complete use your wash bottle (filled with DI) to rinse the residual precipitate from the beaker and the stirring rod into the funnel. a 9. After all the liquid has drained from the funnel very carefully press the top edges of the filter paper together and remove it from the funnel. Place the filter paper in the oven @ 100 C for 20 minutes. 10. Allow to air dry overnight. 11. The next day, record the mass of the filter paper and its contents to the nearest +0.0001 g on the same analytical balance. mass (g) mass of Empty 250 ml beaker (m1) 98.3875 mass of 250 mL beaker + Unknown (m2) 98.7000 mass of Unknown (m2-mi) = (m3) 0.3125 mass of Whatman filler No. 44 (m) 0.7369 mass of Whatman filter No.44 + BaSO4(ms) 1.3448 mass of BaSO4(ms-ma) = (m6) 0.6079 Gtx m6 = (m) 0.2553 % SO42- in Unknown S (mz/m3) 81.70 U