Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Introduction Temperature is a measure of the average speed of the molecules in a substance. The hotter the substance, the faster the molecules are moving.



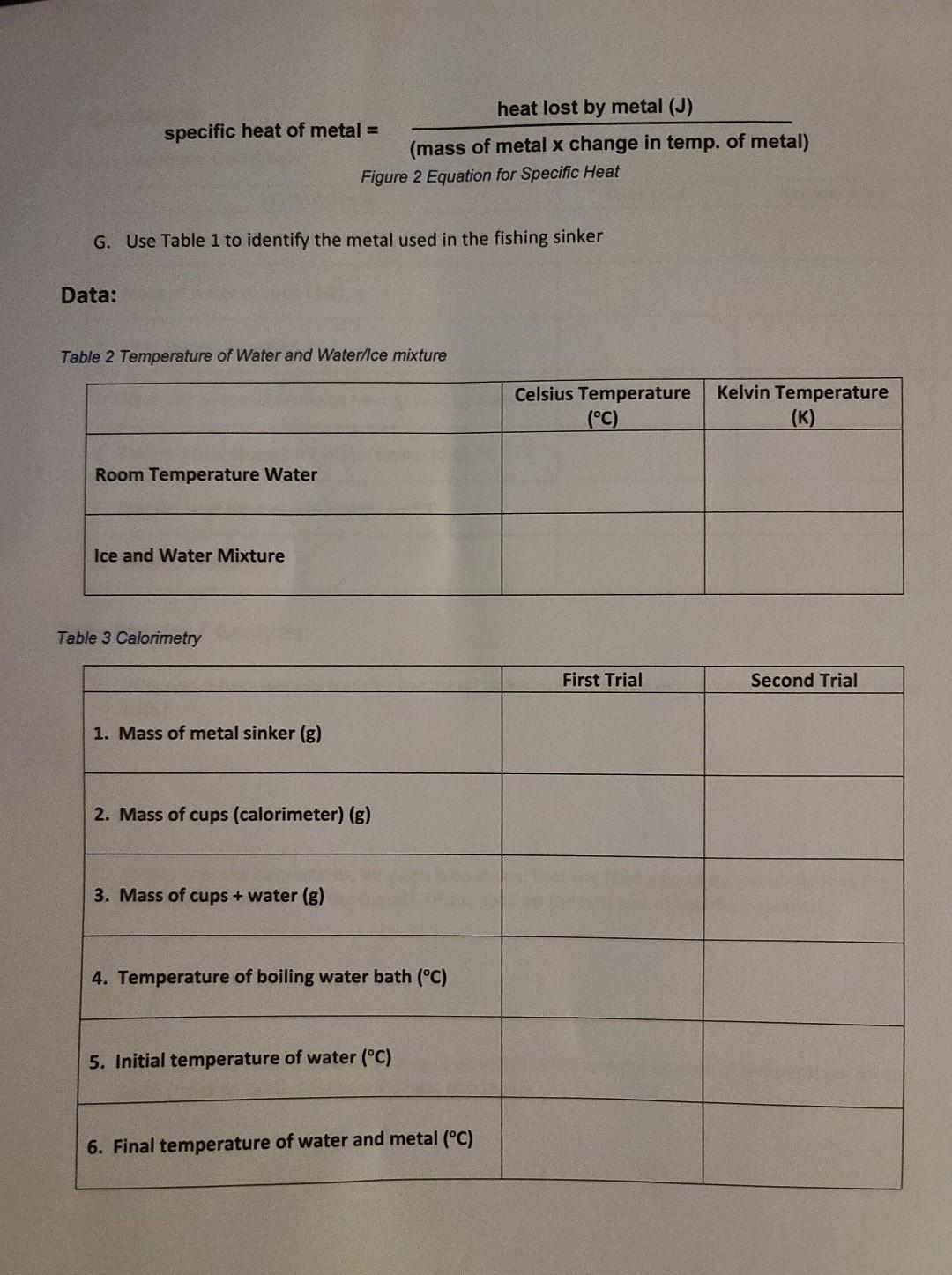
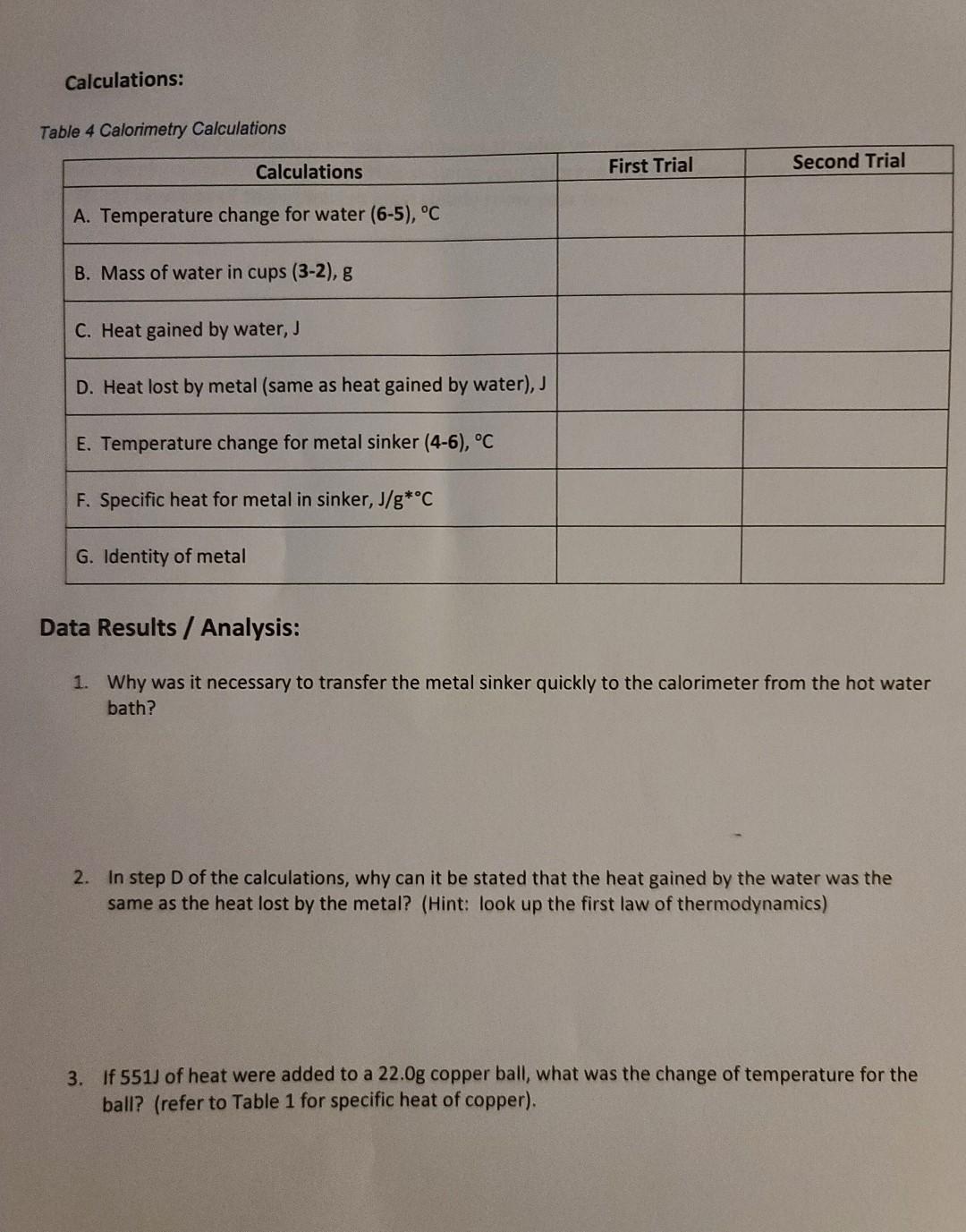
Introduction Temperature is a measure of the average speed of the molecules in a substance. The hotter the substance, the faster the molecules are moving. There are three temperature scales that are used: Fahrenheit, Celsius, and Kelvin. Fahrenheit is the scale commonly used in the United States for both weather and cooking, while Celsius is used throughout most of the rest of the world. The Kelvin, or absolute, temperature scale, is used exclusively in science. You can easily convert between the scales using the following formulas: To convert Celsius to Fahrenheit: TF=1.8(Tc)+32 To convert Fahrenheit to Celsius: TC=(TF32)/1.8 To convert Celsius to Kelvin: Tk=TC+273 For example, the boiling point of water is 212F. To convert that to Celsius: (21232)/1.8=100.C To convert that temperature to Kelvin: 100C+273=373K Different substances will require different amounts of heat to raise their temperature. For example, it takes over 10 times the amount of heat to raise 1 gram of water by 1C in temperature compared to same amount of copper. That is why metal pots and pans heat up very quickly on the stove while wate takes a long time to heat up. The term specific heat refers to the amount of heat (measured in Joules, J) required to raise 1 gram of a substance by 1 degree Celsius. Table 1 gives a list of specific heat valu for a variety of substances. Table 1 Specific Heat for Selected Substances The amount of heat absorbed by a substance depends on how much there is (mass), the temperature change, and the specific heat for the substance. For example, to calculate the amount of heat absorb by 30.0g of water that is heated from 25.0C to 74.0C, we use this formula: Heat =( mass )( specific heat )( change in temperature ) Heat =(30.0g)(4.184J/gC)(74.025.0G)=6150J or 6.15kJ Equipment: - Large metal fishing sinker (2-4 ounces) - String (about 6 inches) - Graduated cylinder - Weighing balance / scale - A drinking glass or cup - 2 Styrofoam cups - Cardboard square (to cover Styrofoam cups) with hole for thermometer - Laboratory thermometer (degrees Celsius) - Small metal pot - Heat source (stove top) - Water - Ice - Camera/smart phone Setup: In this experiment you will find the specific heat for a metal fishing sinker. You will heat the sinker in boiling water, then transfer that hot sinker to room temperature water in a calorimeter (Styrofoam cups, cardboard top, thermometer). See Figure 1 for calorimeter photo. By measuring the change in temperature in the water, and finding the mass of the sinker and the water, you will be able to calculate the specific heat and identify of the metal used in the sinker. Procedure: Temperature 1. Be sure to wear your safety goggles. 2. Observe the markings on the laboratory thermometer. Most will have lines indicating 1C increments, meaning you can estimate to the closest 0.1C (between the lines). 3. Fill your glass or cup about 1/3 full with room temperature water. Place your thermometer (bulb end) into the water and wait until the temperature has remained constant, usually about 2 minutes. Record the temperature in Table 2. 4. Now add approximately an equivalent amount of ice to the glass/cup and record the temperature of the mixture in Table 2. 5. Convert each temperature to Kelvin and record in the third column of Table 2. Specific Heat 1. Be sure to wear your safety goggles. 2. Find the mass of the metal fishing sinker (in grams) using your balance (be sure to zero the balance first). Record the mass in Table 3. 3. Tie the string to the fishing sinker. 4. Fill the small metal pot about 1/2 to 3/4 full with water. 5. Gently lower the metal sinker into the pot, keeping the string out of the water (you will use it remove the hot sinker later). If the water level doesn't fully cover the sinker, add more water. 6. Place the pot of water and sinker on the stove or heat source and heat to boiling. Be careful n to let the string touch the heat source or it may catch fire. Allow the water to boil for 10 minutes. 7. While the water is heating, place one Styrofoam cup inside the other. Find their mass on the balance and record in Table 3 . 8. Place about 50mL room temperature water (using your graduated cylinder) inside the cups anc find the mass of the cups and water on your balance. Record in Table 3. Make sure there is enough water to cover the metal sinker which will be placed in the cups later. Add more water and if necessary before finding the mass. 9. Measure the temperature of the water in the Styrofoam cups and record as initial temperature in Table 3. 10. Measure the temperature of the boiling water in the pot after it has boiled for 10 minutes and record in Table 3. 11. Turn off the heat source / stove top. 12. Carefully remove the sinker out of the hot water using the string (don't burn yourself on the hot water!) and quickly place in the water inside the Styrofoam cups. Immediately place the cover on the calorimeter (Styrofoam cups) with the thermometer in the hole and gently swirl the cup. 13. Using the thermometer, record the highest temperature reached by the water. Record this as the final temperature in Table 3. This is the final temperature for both the water in the calorimeter and the metal sinker. 14. Repeat steps 4 through 13 for the second trial and record your date in Table 3. Calculations A. Calculate the temperature change for the water in the calorimeter (6-5 in data table) B. Calculate the mass of the water in the cup (3-2 in data table) C. Calculate the heat gained by the water: heat = (mass water in cup) (4.184J/gC)( temperature change of water) D. NOTE: The heat calculated in step 3 is equal to the heat LOST by the metal sinker E. Calculate the temperature change for the metal sinker (4-6 in data table) F. Calculate the specific heat for the metal in the sinker using the following equation: specificheatofmetal=(massofmetalxchangeintemp.ofmetal)heatlostbymetal(J) Figure 2 Equation for Specific Heat G. Use Table 1 to identify the metal used in the fishing sinker Data: Table 2 Temperature of Water and Water/lce mixture Table 3 Calorimetry Calculations: Table 4 Calorimetry Calculations Data Results / Analysis: 1. Why was it necessary to transfer the metal sinker quickly to the calorimeter from the hot water bath? 2. In step D of the calculations, why can it be stated that the heat gained by the water was the same as the heat lost by the metal? (Hint: look up the first law of thermodynamics) 3. If 551J of heat were added to a 22.0g copper ball, what was the change of temperature for the ball? (refer to Table 1 for specific heat of copper). Introduction Temperature is a measure of the average speed of the molecules in a substance. The hotter the substance, the faster the molecules are moving. There are three temperature scales that are used: Fahrenheit, Celsius, and Kelvin. Fahrenheit is the scale commonly used in the United States for both weather and cooking, while Celsius is used throughout most of the rest of the world. The Kelvin, or absolute, temperature scale, is used exclusively in science. You can easily convert between the scales using the following formulas: To convert Celsius to Fahrenheit: TF=1.8(Tc)+32 To convert Fahrenheit to Celsius: TC=(TF32)/1.8 To convert Celsius to Kelvin: Tk=TC+273 For example, the boiling point of water is 212F. To convert that to Celsius: (21232)/1.8=100.C To convert that temperature to Kelvin: 100C+273=373K Different substances will require different amounts of heat to raise their temperature. For example, it takes over 10 times the amount of heat to raise 1 gram of water by 1C in temperature compared to same amount of copper. That is why metal pots and pans heat up very quickly on the stove while wate takes a long time to heat up. The term specific heat refers to the amount of heat (measured in Joules, J) required to raise 1 gram of a substance by 1 degree Celsius. Table 1 gives a list of specific heat valu for a variety of substances. Table 1 Specific Heat for Selected Substances The amount of heat absorbed by a substance depends on how much there is (mass), the temperature change, and the specific heat for the substance. For example, to calculate the amount of heat absorb by 30.0g of water that is heated from 25.0C to 74.0C, we use this formula: Heat =( mass )( specific heat )( change in temperature ) Heat =(30.0g)(4.184J/gC)(74.025.0G)=6150J or 6.15kJ Equipment: - Large metal fishing sinker (2-4 ounces) - String (about 6 inches) - Graduated cylinder - Weighing balance / scale - A drinking glass or cup - 2 Styrofoam cups - Cardboard square (to cover Styrofoam cups) with hole for thermometer - Laboratory thermometer (degrees Celsius) - Small metal pot - Heat source (stove top) - Water - Ice - Camera/smart phone Setup: In this experiment you will find the specific heat for a metal fishing sinker. You will heat the sinker in boiling water, then transfer that hot sinker to room temperature water in a calorimeter (Styrofoam cups, cardboard top, thermometer). See Figure 1 for calorimeter photo. By measuring the change in temperature in the water, and finding the mass of the sinker and the water, you will be able to calculate the specific heat and identify of the metal used in the sinker. Procedure: Temperature 1. Be sure to wear your safety goggles. 2. Observe the markings on the laboratory thermometer. Most will have lines indicating 1C increments, meaning you can estimate to the closest 0.1C (between the lines). 3. Fill your glass or cup about 1/3 full with room temperature water. Place your thermometer (bulb end) into the water and wait until the temperature has remained constant, usually about 2 minutes. Record the temperature in Table 2. 4. Now add approximately an equivalent amount of ice to the glass/cup and record the temperature of the mixture in Table 2. 5. Convert each temperature to Kelvin and record in the third column of Table 2. Specific Heat 1. Be sure to wear your safety goggles. 2. Find the mass of the metal fishing sinker (in grams) using your balance (be sure to zero the balance first). Record the mass in Table 3. 3. Tie the string to the fishing sinker. 4. Fill the small metal pot about 1/2 to 3/4 full with water. 5. Gently lower the metal sinker into the pot, keeping the string out of the water (you will use it remove the hot sinker later). If the water level doesn't fully cover the sinker, add more water. 6. Place the pot of water and sinker on the stove or heat source and heat to boiling. Be careful n to let the string touch the heat source or it may catch fire. Allow the water to boil for 10 minutes. 7. While the water is heating, place one Styrofoam cup inside the other. Find their mass on the balance and record in Table 3 . 8. Place about 50mL room temperature water (using your graduated cylinder) inside the cups anc find the mass of the cups and water on your balance. Record in Table 3. Make sure there is enough water to cover the metal sinker which will be placed in the cups later. Add more water and if necessary before finding the mass. 9. Measure the temperature of the water in the Styrofoam cups and record as initial temperature in Table 3. 10. Measure the temperature of the boiling water in the pot after it has boiled for 10 minutes and record in Table 3. 11. Turn off the heat source / stove top. 12. Carefully remove the sinker out of the hot water using the string (don't burn yourself on the hot water!) and quickly place in the water inside the Styrofoam cups. Immediately place the cover on the calorimeter (Styrofoam cups) with the thermometer in the hole and gently swirl the cup. 13. Using the thermometer, record the highest temperature reached by the water. Record this as the final temperature in Table 3. This is the final temperature for both the water in the calorimeter and the metal sinker. 14. Repeat steps 4 through 13 for the second trial and record your date in Table 3. Calculations A. Calculate the temperature change for the water in the calorimeter (6-5 in data table) B. Calculate the mass of the water in the cup (3-2 in data table) C. Calculate the heat gained by the water: heat = (mass water in cup) (4.184J/gC)( temperature change of water) D. NOTE: The heat calculated in step 3 is equal to the heat LOST by the metal sinker E. Calculate the temperature change for the metal sinker (4-6 in data table) F. Calculate the specific heat for the metal in the sinker using the following equation: specificheatofmetal=(massofmetalxchangeintemp.ofmetal)heatlostbymetal(J) Figure 2 Equation for Specific Heat G. Use Table 1 to identify the metal used in the fishing sinker Data: Table 2 Temperature of Water and Water/lce mixture Table 3 Calorimetry Calculations: Table 4 Calorimetry Calculations Data Results / Analysis: 1. Why was it necessary to transfer the metal sinker quickly to the calorimeter from the hot water bath? 2. In step D of the calculations, why can it be stated that the heat gained by the water was the same as the heat lost by the metal? (Hint: look up the first law of thermodynamics) 3. If 551J of heat were added to a 22.0g copper ball, what was the change of temperature for the ball? (refer to Table 1 for specific heat of copper)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


