Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Iodine- 131 is a radioactive isotope that is used to diagnose and treat some forms of thyroid cancer. Iodine- 131 decays to xenon-131, according to
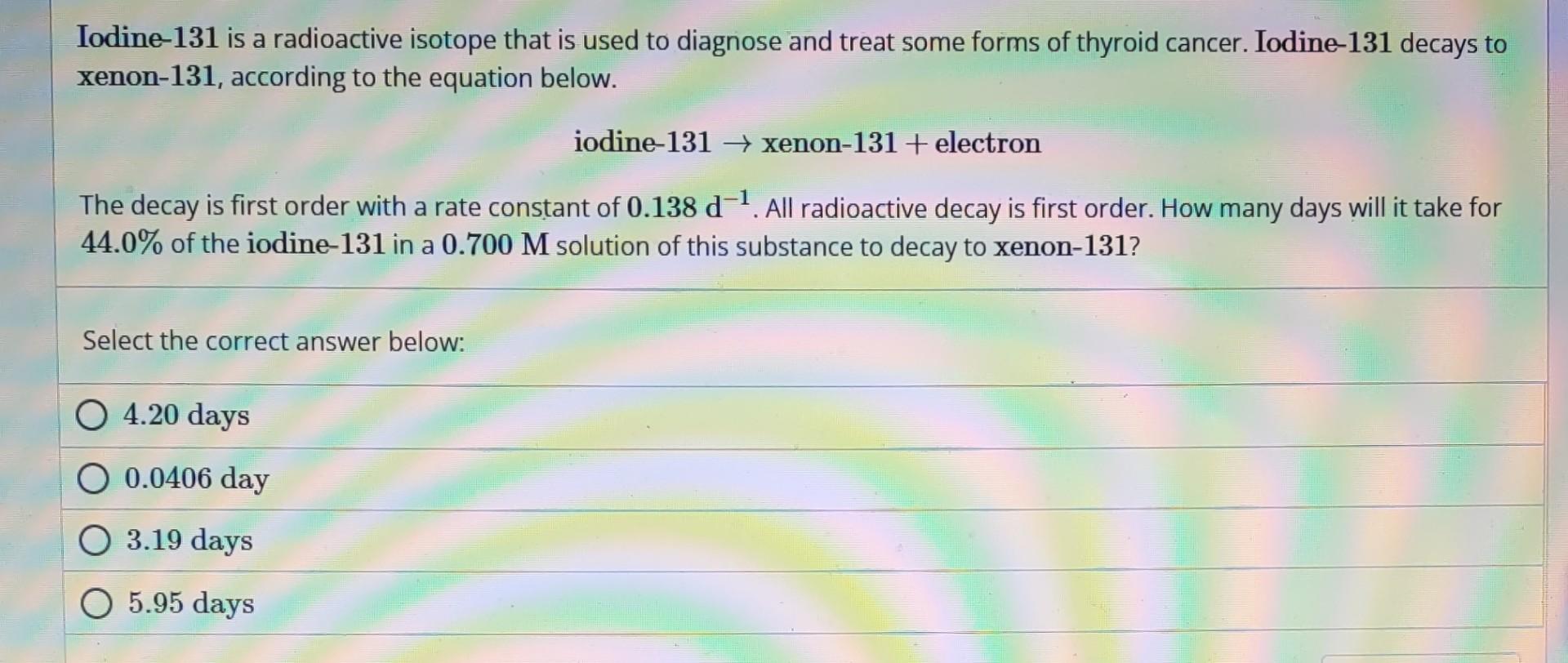
Iodine- 131 is a radioactive isotope that is used to diagnose and treat some forms of thyroid cancer. Iodine- 131 decays to xenon-131, according to the equation below. iodine- 131 xenon- 131+ electron The decay is first order with a rate constant of 0.138d1. All radioactive decay is first order. How many days will it take for 44.0% of the iodine- 131 in a 0.700M solution of this substance to decay to xenon- 131 ? Select the correct answer below: 4.20 days 0.0406 day 3.19 days 5.95 days
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


