Answered step by step
Verified Expert Solution
Question
1 Approved Answer
is this correct? Synthesis gas is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen,
is this correct? 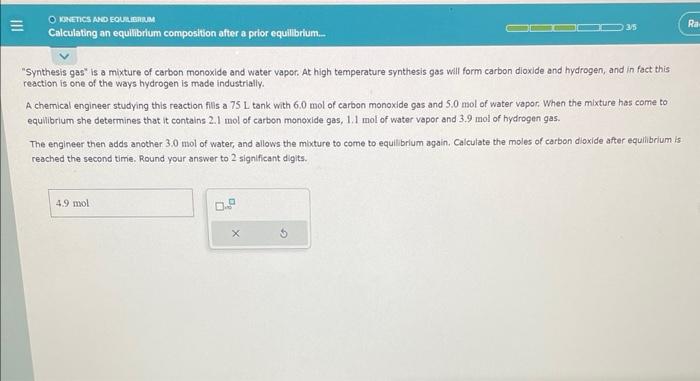
"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction filis a 75L tank with 6.0 mol of carbon monoxide gas and 5.0 mol of water vapor. When the mixture has come to equlibrium-she determines that it contains 2.1 mol of carbon monoxide gas, 1.1 mol of water vapor and 3.9 mol of hydrogen gas. The engineer then adds another 3.0 mol of water, and allows the moture to come to equilibrlum again, Calculate the moles of carbon dioxide after equilibrium is reached the second time. Round your answer to 2 significant digits 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


