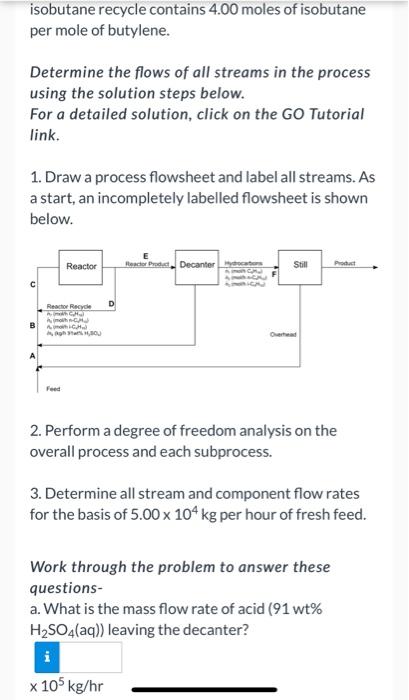
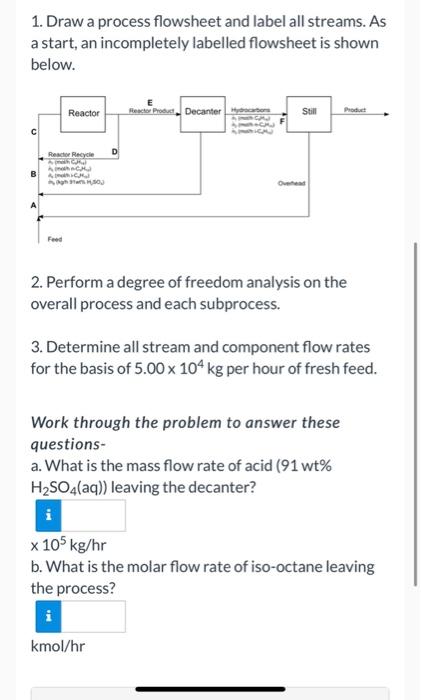
Iso-octane is produced in the reaction of isobutane and butylene in an emulsion with concentrated sulfuric acid: i-C4H10 + C4H8 - i-C3H18 Fresh feed to the process flows at a rate of 5.00 x 104 kg/hr and contains 30.0 mol% isobutane, 30.0 mol% butylene, and 40.0 mol% n-butane, which is chemically inert in this process. The fresh feed combines with three separate recycle streams, and the combined stream enters the reactor. Essentially all of the butylene fed to the reactor is consumed. A portion of the reactor effluent is recycled to the reactor inlet and the remainder passes to a decanter, in which the aqueous (sulfuric acid) and hydrocarbon phases are allowed to separate. The acid is recycled to the reactor, and the hydrocarbons pass to a distillation column. The bottoms product from the column contains iso- octane and n-butane, and the overhead product or distillate, which is recycled to the reactor, contains only isobutane. The stream entering the reactor contains 200.0 moles of isobutane per mole of butylene, and 3.00 kg of 91 wt% H2SO4(aq) per kg of hydrocarbon (3.00 kg of acid solution per kg of total hydrocarbon). The stream obtained by combining the fresh feed and isobutane recycle contains 400 males of isobutane isobutane recycle contains 4.00 moles of isobutane per mole of butylene. Determine the flows of all streams in the process using the solution steps below. For a detailed solution, click on the GO Tutorial link. 1. Draw a process flowsheet and label all streams. As a start, an incompletely labelled flowsheet is shown below. Reactor Reacher Product Decanterye Still Pochet c Reactor Recyde D 2. Perform a degree of freedom analysis on the overall process and each subprocess. 3. Determine all stream and component flow rates for the basis of 5.00 x 104 kg per hour of fresh feed. Work through the problem to answer these questions- a. What is the mass flow rate of acid (91 wt% H2SO4(aq)) leaving the decanter? x 105 kg/hr 1. Draw a process flowsheet and label all streams. As a start, an incompletely labelled flowsheet is shown below. Reactor Reactor Product Decanter to Still Prol Reactor Recycle D SO 2. Perform a degree of freedom analysis on the overall process and each subprocess. 3. Determine all stream and component flow rates for the basis of 5.00 x 104 kg per hour of fresh feed. Work through the problem to answer these questions- a. What is the mass flow rate of acid (91 wt% H2SO4(aq)) leaving the decanter? x 105 kg/hr b. What is the molar flow rate of iso-octane leaving the process? kmol/hr