Question
isotope 24 Mg 25 Mg 26Mg mass (amu) percent abundance 23.985042 78.99% 24.985837 10.00% 25.982593 11.01% What is the value: atomic massx fractional abundance
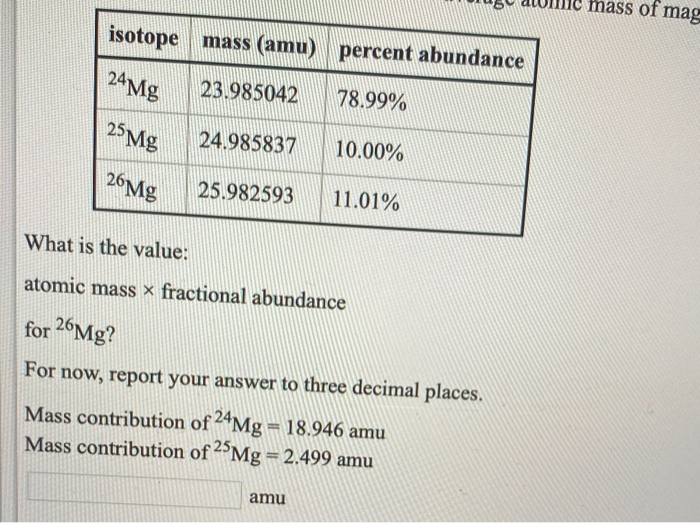
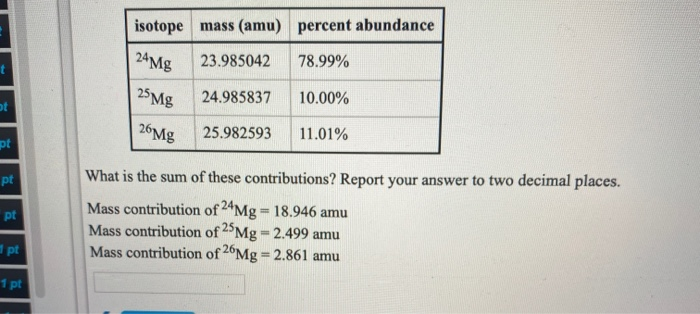
isotope 24 Mg 25 Mg 26Mg mass (amu) percent abundance 23.985042 78.99% 24.985837 10.00% 25.982593 11.01% What is the value: atomic massx fractional abundance for 26Mg? For now, report your answer to three decimal places. Mass contribution of 24Mg = 18.946 amu Mass contribution of 25Mg = 2.499 amu amu ass of mag t ot pt pt pt pt 1 pt isotope mass (amu) percent abundance 24 Mg 78.99% 25 Mg 26Mg 23.985042 24.985837 25.982593 10.00% 11.01% What is the sum of these contributions? Report your answer to two decimal places. Mass contribution of 24Mg = 18.946 amu Mass contribution of 25 Mg = 2.499 amu Mass contribution of 26Mg = 2.861 amu
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Statistics The Exploration & Analysis Of Data
Authors: Roxy Peck, Jay L. Devore
7th Edition
0840058012, 978-0840058010
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



