Answered step by step
Verified Expert Solution
Question
1 Approved Answer
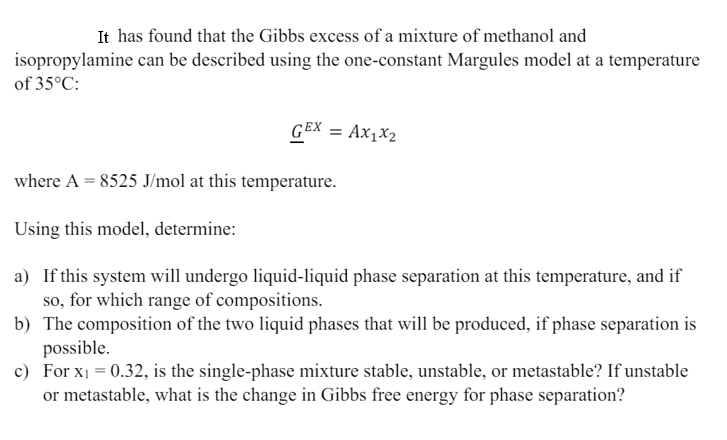
It has found that the Gibbs excess of a mixture of methanol and isopropylamine can be described using the one - constant Margules model at
It has found that the Gibbs excess of a mixture of methanol and
isopropylamine can be described using the oneconstant Margules model at a temperature
of :
where at this temperature.
Using this model, determine:
a If this system will undergo liquidliquid phase separation at this temperature, and if
so for which range of compositions.
b The composition of the two liquid phases that will be produced, if phase separation is
possible.
c For is the singlephase mixture stable, unstable, or metastable? If unstable
or metastable, what is the change in Gibbs free energy for phase separation?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


