Question
It includes wet vapor of R717 with a quality of 0.2 at a temperature of 15oC in a frictionless and well insulated piston-cylinder system shown
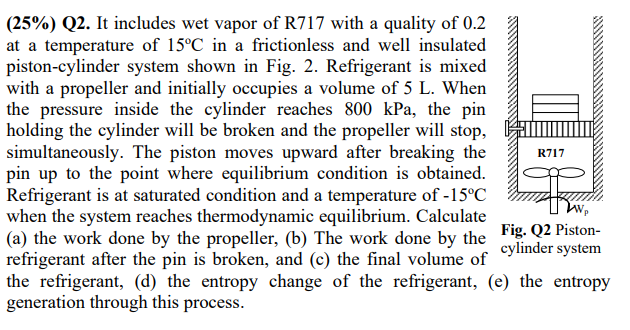 It includes wet vapor of R717 with a quality of 0.2 at a temperature of 15oC in a frictionless and well insulated piston-cylinder system shown in Fig. 2. Refrigerant is mixed with a propeller and initially occupies a volume of 5 L. When the pressure inside the cylinder reaches 800 kPa, the pin holding the cylinder will be broken and the propeller will stop, simultaneously. The piston moves upward after breaking the pin up to the point where equilibrium condition is obtained. Refrigerant is at saturated condition and a temperature of -15oC when the system reaches thermodynamic equilibrium. Calculate (a) the work done by the propeller, (b) The work done by the refrigerant after the pin is broken, and (c) the final volume of the refrigerant, (d) the entropy change of the refrigerant, (e) the entropy generation through this process.
It includes wet vapor of R717 with a quality of 0.2 at a temperature of 15oC in a frictionless and well insulated piston-cylinder system shown in Fig. 2. Refrigerant is mixed with a propeller and initially occupies a volume of 5 L. When the pressure inside the cylinder reaches 800 kPa, the pin holding the cylinder will be broken and the propeller will stop, simultaneously. The piston moves upward after breaking the pin up to the point where equilibrium condition is obtained. Refrigerant is at saturated condition and a temperature of -15oC when the system reaches thermodynamic equilibrium. Calculate (a) the work done by the propeller, (b) The work done by the refrigerant after the pin is broken, and (c) the final volume of the refrigerant, (d) the entropy change of the refrigerant, (e) the entropy generation through this process.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


