Question
It is an electrophilic aromatic substitution lab where we prepared 1-chloro-2,4-dinitrobenzene using chlorobenzene, nitric acid and sulfuric acid. -Write an equation showing the role of
It is an electrophilic aromatic substitution lab where we prepared 1-chloro-2,4-dinitrobenzene using chlorobenzene, nitric acid and sulfuric acid.
-Write an equation showing the role of sulfuric acid in the nitration reaction. H2SO4 pKa = -3; HNO3 pKa = -1.64
- Compare the 1H NMR spectra of the product with those of probable byproducts. Assign the resonances to particular H-atoms and explain your assignment. Compare the IR- spectra of chlorobenzene and your product. Note any significant changes.
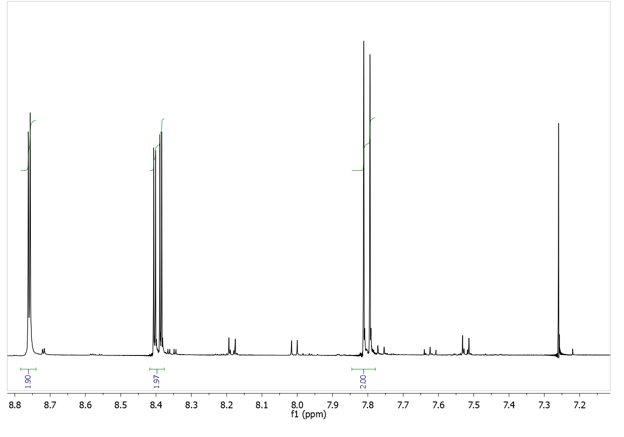
- How does the introduction of two nitro groups into chlorobenzene allow nucleophilic aromatic substitution?
Ju . Ju Fost F-26 rooz 8.8 8.7 8.6 8.5 8.4 8.3 8.2 8.1 7.8 7.7 7.6 7.5 7.4 7.3 7.2 8.0 7.9 f1 (ppm) Ju . Ju Fost F-26 rooz 8.8 8.7 8.6 8.5 8.4 8.3 8.2 8.1 7.8 7.7 7.6 7.5 7.4 7.3 7.2 8.0 7.9 f1 (ppm)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


