Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It is assumed that 10.00mL TRIS sample (TRIS; tris-(hydroxymethyl)aminomethane, (HOCH2)3CNH2 ) with a concentration of 24g TRIS per L sample is titrated with 0.100M hydrochloric
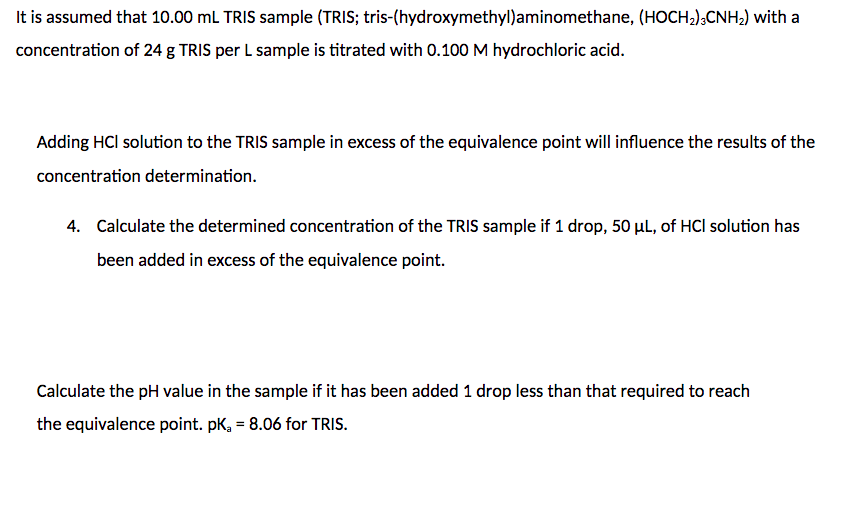 It is assumed that 10.00mL TRIS sample (TRIS; tris-(hydroxymethyl)aminomethane, (HOCH2)3CNH2 ) with a concentration of 24g TRIS per L sample is titrated with 0.100M hydrochloric acid. Adding HCl solution to the TRIS sample in excess of the equivalence point will influence the results of the concentration determination. 4. Calculate the determined concentration of the TRIS sample if 1 drop, 50L, of HCl solution has been added in excess of the equivalence point. Calculate the pH value in the sample if it has been added 1 drop less than that required to reach the equivalence point. pKa=8.06 for TRIS
It is assumed that 10.00mL TRIS sample (TRIS; tris-(hydroxymethyl)aminomethane, (HOCH2)3CNH2 ) with a concentration of 24g TRIS per L sample is titrated with 0.100M hydrochloric acid. Adding HCl solution to the TRIS sample in excess of the equivalence point will influence the results of the concentration determination. 4. Calculate the determined concentration of the TRIS sample if 1 drop, 50L, of HCl solution has been added in excess of the equivalence point. Calculate the pH value in the sample if it has been added 1 drop less than that required to reach the equivalence point. pKa=8.06 for TRIS Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


