Question: it is talking about thw book but i need a dumber version and example with the work i actually do Balance the following equation using


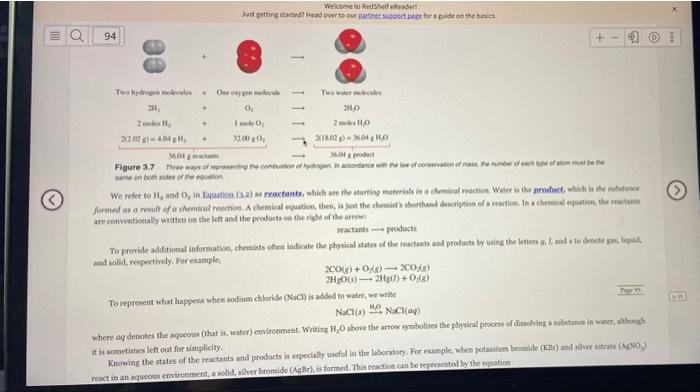
Balance the following equation using the method outlined in Section 3.7. (If the coefficient is 1, you must enter a 1.) C4H10 + Cl2 C4H4C16 + H2 D Question 22 4 pts Balance the following equation using the method outlined in Section 3.7. (If the coefficient is 1, you must enter a 1.) C2H26 + 02 CO2 + HO 94 molar mass is about 70 92 3.7 Chemical Reactions and Chemical Equations Having discussed the masses of atoms and molecules, we turn next to what happens to atoms and molecules in a chemical reaction, a process in which a substance (or substances) is changed into one or more new substances. To communicate with one another about chemical reactions, chemists have devised a standard way to represent them using chemical equations. A chemical equation uses chemical symbols to show that happens during a chemical reaction. In this section, Page 94 we will learn how to write chemical equations and balance them. Writing Chemical Equations Consider what happens when hydrogen gas (H.) burns in air (which contains onygen, O.) to form water (H,0). This reaction can be represented by the chemical equation H+0.-H.0 (32) where the "plus" sign means "reacts with and the arrow means to yield. Thus, this symbolic expression can be read: "Molecular hydrogen reacts with molecular oxygen to yield water." The reaction is assumed to proceed from left to right as the arrow indicates We use the law of conservation of mass as our guide in balancing chemical equations. Equation (3-2) is not complete, however, because there are twice as many oxygen atoms on the left side of the arrow (two) as on the right side (one). To conform with the law of conservation of mass, there must be the same number of each type of atom on both sides of the arrow-that is, we must have as many atoms after the reaction ends as we did before it started. We can balance Equation (3-2) by placing the appropriate coefficient (e in this case) in front of H, and H.0: 2H, +0; -24,0 When the recent is 1, as in the case of it is not shown. This balanced chemical equation shows that two hydrogen molecules can combine or react with one oxygen molecule to form two water molecules" (Egure 3,7). Because the ratio of the number of molecules is equal to the ratio of the number of moles, the equation can also be read as moles of hydrogen molecules react with 1 mole of oxygen molecules to produce 2 moles of water molecules." We know the mass of a mole of each of these substances, so we can also interpret the equation as 4,04 g of H, react with 32.00 g of O, to give 36.04 8 of H.O." These three ways of reading the equation are summarised in Eikute: 3.7. Welcome to RedShelldet Just getting started Head over to our page for pade on the basics 94 + @ Two meteostes. Detay Two 0 2.0 2 + IO 2 2000 21.02 MOLHO 16.04 360 de Figure 3.7 Thways of the combustion des accordance with coche samento We refer to H, and in Equation (S.2) reactants, which are the starting materials in a chemical action Water is the product, which is the substan formed as a result of a chemical reaction. A chemical equation, then, is just the thema's shorthand description of a reaction. In chemical equation, the recta are conventionally written on the left and the protects on the right of the reactants products To provide additional information, chemists often indicate the physical states of the reactants and products by using the best and to dete, liguld, and solid, respectively. For example, 200) + O) 20.6) 2H00) 2H(+0,6) To represent what happens when sodium chloride (act) is added to water, we write NOCH NICI where a denotes the aqueous (that is, water) environment. Writing 1,0 above the arrow symbolines the physical process of disolving a substance in water, hoch it is sometimes left out for simplicity, Knowing the states of the reactants and products is especially useful in the laboratory. For example, when potassium bromide (KB) and silver nitrate (NO) react in an aqueous environment, a solid, silver bromide (Br), is formed. This reaction can be represented by the equation Welcome to HedShelf Reader Just getting started Head over to our other support pret for a wide on the basics denotes the aqueous (that is, watet) environment. Writing 1,0 above the arrow symbolines the physical process of dissolving a sobrtance in 95 Jetimes left out for simplicity Knowing the states of the reactants and products is especially useful in the laboratory. For cumple, wbien potassium brutmide (Kor) and uliver nitrate (AgNO, react in an aqueous environment, a solid, silver bromide (Be), is formed. This reaction can be represented by the equation KBr(aq) + AgNO, (aq) - KNO,(aq) + AgBr(s) Studio Spot Student data indicate you may struggle with interpreting chemical equations Access your book for Learning Resources on the topic If the physical states of reactants and products are not given in uninformed person might try to bring about the reaction by mixing olid Be with solid ANO, These solids would react very slowly or not at all. Imagining the process on the microscopic level, we can understand that for a product like silver bromide to form the 1st and Br fons would have to come in contact with each other. However, these ions are locked in place in their solid compounds and have little mobility. Here is an example of how we explain a phenomenon by thinking about what happens at the molecular level, as discussed in Section 1.2.) Balancing Chemical Equations Suppose we want to write an equation to describe a chemical reaction that we have just carried out in the laboratory. How should we go about doing it? Because we know the identities of the reactants, we can write their chemical formulas. The identities of products are more difficult to establish For simple reactions it is often possible to guess the product(s). For more complicated reactions involving three or more products, chemists may need to perform further tests to establish the presence of specific compounds. Once we have identified all the reactants and products and have written the correct formulas for them, we assemble them in the conventional sequence-factants on the left separated by an arrow from products on the right. The equation written at this point is likely to be unbalanced that is, the number of each type of atom on one side of the arrow differs from the number on the other side. In general, we can balance a chemical equation by the following steps: 1. Identify all reactants and products and write their correct formulas on the left side and right side of the equation, respectively 2. Begin balancing the equation by trying different coefficients to make the number of atoms of each element the same on both sides of the equation. We can change the coefficients (the numbers preceding the formulas) but not the subscripts (the numbers within formulas). Changing the subscripts would change the identity of the substance. For example, 2NO, means "two molecules of nitrogen dioxide," but if we double the subscripts, we have N.0, which is the formula of dimitrogen tetroxide, a completely different compound 3. First, look for elements that appear only once on each side of the equation with the same number of atoms on each side: The formulas containing these elements must have the same coefficient. Therefore, there is no need to adjust the coefficients of these elements at this point. Next, look for elements that appear only once Welcome to edheftet Just getting started Head over to our Doctora guide on the back t have the same coefficient. Therefore, there is no need to be the cofficient of the elements at this poin. Netbook for elements that of 96 hach side of the equation but in unequal numbers of atom. Balance the elements. Finally, balance elements that appear in two more form + - wide of the equation Check your balanced equation to be sure that you have the same total number of each type of atoms on both sides of the equat > Hating point wygen when a wood McGraw-Hill Education Ken Kamp Let's comunider a specific example. In the laboratory, small amounts of oxygen pas cu be prepared by beating potassium chlorate (CO). The products we (O) and potassium chloride (KCH). From this information, we write KCIO,KCIO, (For simplicity, we omit the physical states of reactants and products) All three elements (K, C, and appear only one each side of the equation, but only for and al do we have equal numbers of atoms on both sides. Thus, do, and I must have the same coefficient. The next step is to make the number of atoms the same on both sides of the equation. Because there are three Oatoms on the left and two atoms an the right of the equation, we can balance the stoms by placing 2 in front of to, and a 3 in front ofo, 2KCIO, --KCI +30 Finally, we balance the Kand Clatoms by placing as in front of: 2KCIO, -2KCI+ 30, As a final check, we can draw up a balance sheet for the reactants and products where the number in parentheses indicates the number of atoms of each element www Welcome to Red Shelf Reader Just getting started? Head over to our support pose for a guide on the basies Q 97 Mesti Prade CE OIN New web www KCC New Member in the comment C6000, Whende i en billet de petit fe en wollen. Controle e leme C020, 10 The two CL00, NO Mouth Centrum De best they were C+0200, NO There is bases de amor. lo Orgully own Recrudesta cu 010 C+0,000 The tale Berta Product CU ce HO OCH
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


