Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It might be helpful to refer to the following table for questions #1 through #4. Note that each column constitutes a separate DUE: question. Isotope
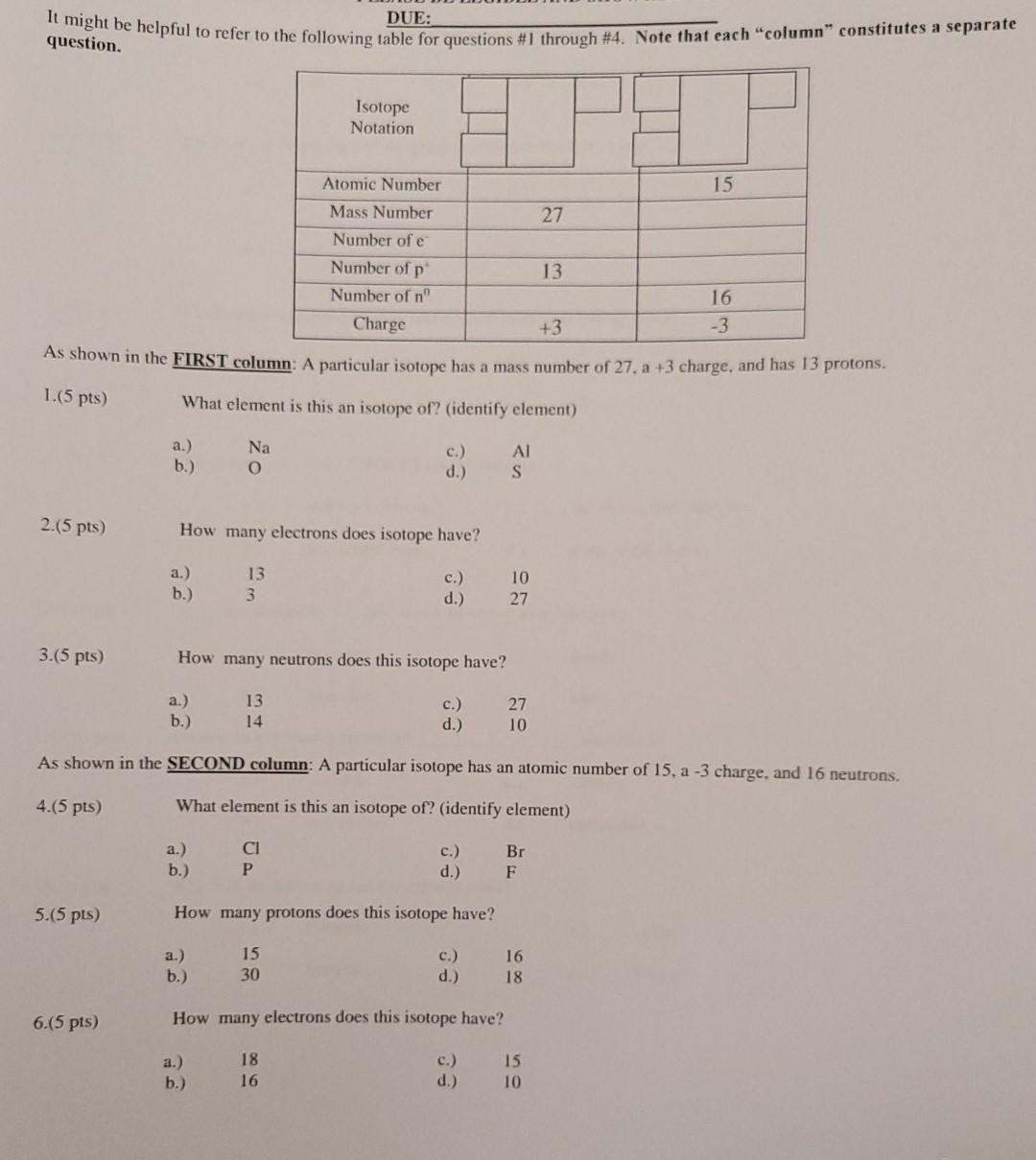

It might be helpful to refer to the following table for questions #1 through #4. Note that each "column" constitutes a separate DUE: question. Isotope Notation Atomic Number 15 Mass Number 27 Number of e Number of p 13 Number of n' 16 Charge +3 -3 As shown in the FIRST column: A particular isotope has a mass number of 27. a +3 charge, and has 13 protons. 1.(5 pts) What element is this an isotope of? (identify element) a.) b.) Na O S d.) 2.(5 pts) How many electrons does isotope have? a.) b.) 13 3 c.) d.) 10 27 3.(5 pts) How many neutrons does this isotope have? a.) b.) 13 14 27 10 d.) As shown in the SECOND column: A particular isotope has an atomic number of 15, a -3 charge, and 16 neutrons. 4.(5 pts) What element is this an isotope of? (identify element) CI P b.) Br F d.) 5.(5 pts) How many protons does this isotope have? 15 30 b.) 16 18 d.) 6.(5 pts) How many electrons does this isotope have? 18 16 c.) d.) 15 10 b.) 7.(10 pts.) Which ion would you not expect to form? a.) F c.) Br b.) S2 d.) K 8. (10 pts.) Which of the following has the smallest atomic size/radius? a.) Li c.) K b.) Na d.) Rb 9. (10 pts.) Which of the following has the greatest effective nuclear charge. Zert? a.) Li .) b.) B d.) O 10. (5 pts.) Which of the following has the highest first ionization energy (IE1)? a.) Li C b.) B d.) 0 11. (5 pts.) One the periodic table, GROUPS are the same as a.) vertical columns c.) the zigzag line/staircase b.) horizontal rows d.) none of the above 12. (10 pts.) are formed by the gain or loss of one or more electrons. a.) isotopes c.) quarks b.) neutrons d.) ions 13. (10 pts) Outermost electron(s) are called electron(s). a.) valuable c.) valence b.) low energy d.) unreactive 14. (10 pt.) Which of the following has the greatest mass? a.) Electron c.) atom b.) neutron d.) Proton
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


