Answered step by step
Verified Expert Solution
Question
1 Approved Answer
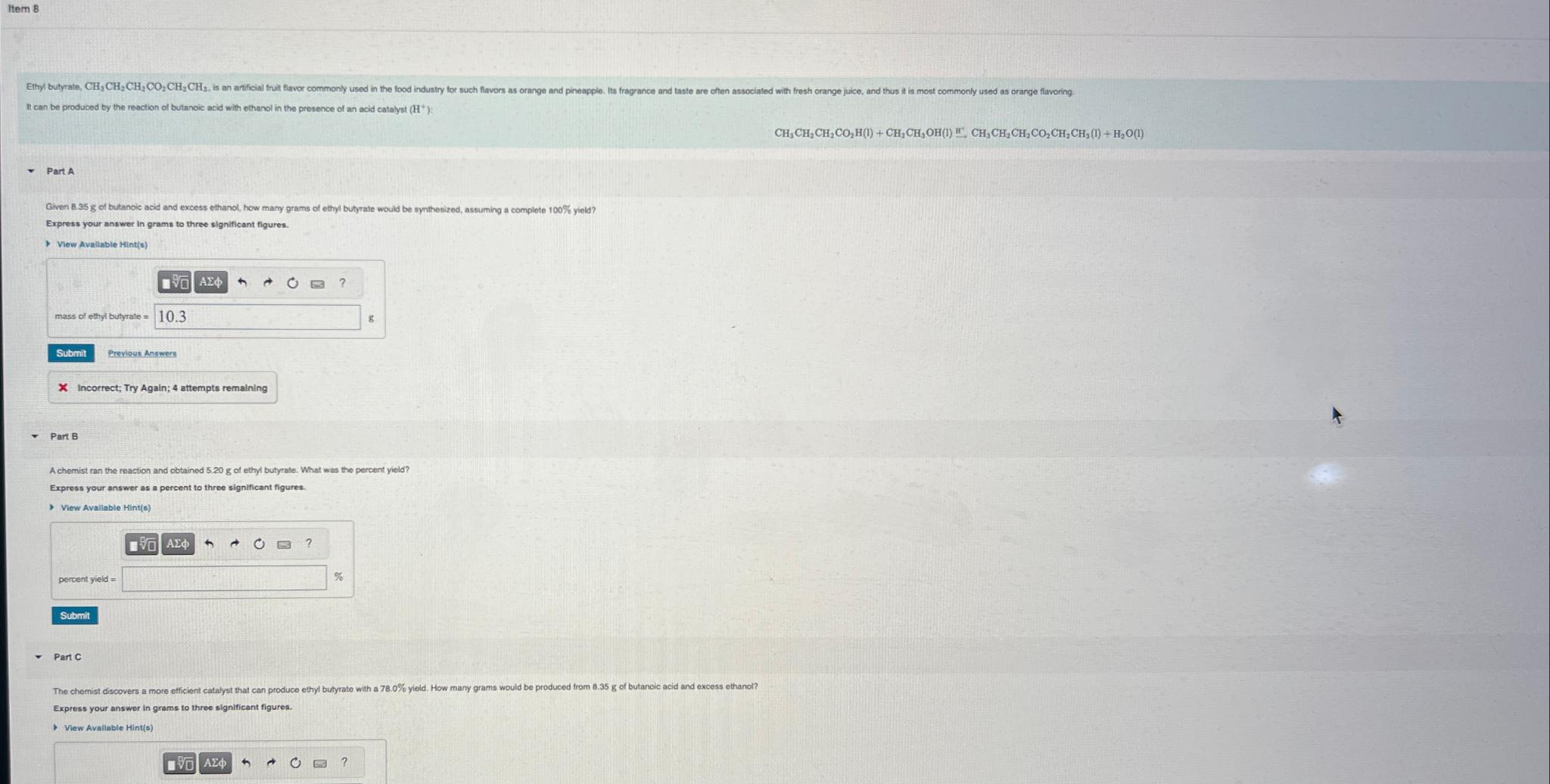
Item 8 It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst ( H + )
Item
It can be produced by the reaction of butanoic acid with ethanol in the presence of an acid catalyst
I
Part A
Given of tutanoic acid and excess ethanol, how many grams of ethyl butyrate would be synthesized, assuming a complete yield?
Express your answer in grams to three significant figures.
View Avallable Hints
Incorrect; Try Again; attempts remaining
Part B
Achemist ran the reaction and obtained of ethyl butyrate. What was the percent yield?
View Avallable Hints
percent yield
Part C
The chemist discovers a more efficient catalyst that can produce ethyl butyrate with a yield. How many grams would be produced from of butanoic acid and excess ethanol?
Express your answer in grams to three significant figures.
View Avaliable Hints
AID
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


