Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It's a matlab equation for chemical engineering. 2.4, Consider the following mixture going into a water-gas shift reactor to make hydrogen for the hydrogen economy.
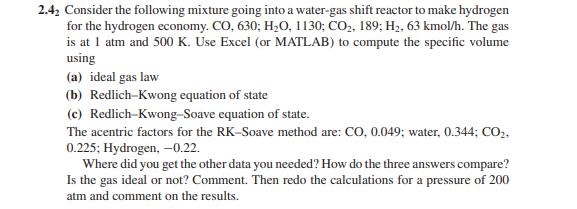 It's a matlab equation for chemical engineering.
It's a matlab equation for chemical engineering.
2.4, Consider the following mixture going into a water-gas shift reactor to make hydrogen for the hydrogen economy. CO, 630; H,O, 1130; CO2, 189; H2, 63 kmol/h. The gas is at 1 atm and 500 K. Use Excel (or MATLAB) to compute the specific volume using (a) ideal gas law (b) Redlich-Kwong equation of state (c) Redlich-Kwong-Soave equation of state. The acentric factors for the RK-Soave method are: CO, 0.049; water, 0.344; CO,. 0.225; Hydrogen, -0.22. Where did you get the other data you needed? How do the three answers compare? Is the gas ideal or not? Comment. Then redo the calculations for a pressure of 200 atm and comment on the results. 2.4, Consider the following mixture going into a water-gas shift reactor to make hydrogen for the hydrogen economy. CO, 630; H,O, 1130; CO2, 189; H2, 63 kmol/h. The gas is at 1 atm and 500 K. Use Excel (or MATLAB) to compute the specific volume using (a) ideal gas law (b) Redlich-Kwong equation of state (c) Redlich-Kwong-Soave equation of state. The acentric factors for the RK-Soave method are: CO, 0.049; water, 0.344; CO,. 0.225; Hydrogen, -0.22. Where did you get the other data you needed? How do the three answers compare? Is the gas ideal or not? Comment. Then redo the calculations for a pressure of 200 atm and comment on the results.
Step by Step Solution
★★★★★
3.45 Rating (142 Votes )
There are 3 Steps involved in it
Step: 1
Ahsweng Specific Uolume RTpm TOTal molar Abw Jate 630...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


