Question
(It's a physical chemistry problem) Consider a well-insulated piston-cylinder assembly shown above. On the 0.05 m2 piston rests two 5000-kg blocks. The initial temperature is
(It's a physical chemistry problem)
Consider a well-insulated piston-cylinder assembly shown above. On the 0.05 m2 piston rests two 5000-kg blocks. The initial temperature is 500.00 K. The ambient pressure is 5 bar. One mole of an ideal gas is contained in the cylinder. The gas is compressed in a process in which another 5000-kg block is added. The molar heat capacity at constant volume(Cv) can be taken to have a constant value of 5/2R and the gravitational constant is 9.81 ms-2
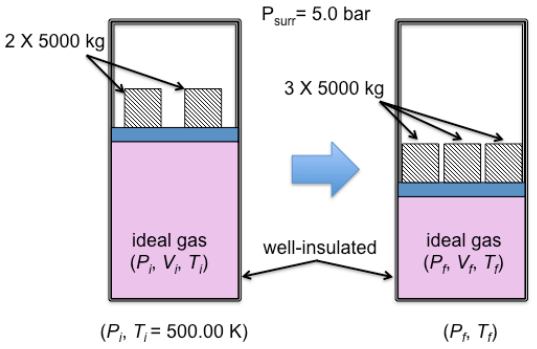
(1) Do you expect the temperature to rise or fall? Explain in a sentence or two.
(The answer in the book is (1) Temperature should rise - convention we have followed is that work done on the system is positive. System is insulated so energy conservation would require the temperature to rise)
Please explain the answer using the formula
Please write neatly so that I can read it easily and write the solution process so that the above answer comes out.
Psurr= 5.0 bar 2 X 5000 kg 3 X 5000 kg ideal gas (P; V;, T) well-insulated ideal gas (PF, V, T1) (Pi, T;= 500.00 K) (PF, T:)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


