Answered step by step
Verified Expert Solution
Question
1 Approved Answer
It's Che 301:Chemical Engineering Thermodynamics course. Can you help me? I will rate your work. Thank you. Problem 5 The triple point of Carbon Dioxide
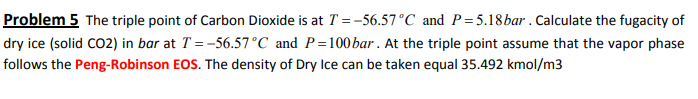
It's Che 301:Chemical Engineering Thermodynamics course. Can you help me? I will rate your work. Thank you.
Problem 5 The triple point of Carbon Dioxide is at T =-56.57C and P=5.18bar . Calculate the fugacity of dry ice (solid CO2) in bar at T = -56.57C and P=100 bar. At the triple point assume that the vapor phase follows the Peng-Robinson EOS. The density of Dry Ice can be taken equal 35.492 kmol/m3Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


