Answered step by step
Verified Expert Solution
Question
1 Approved Answer
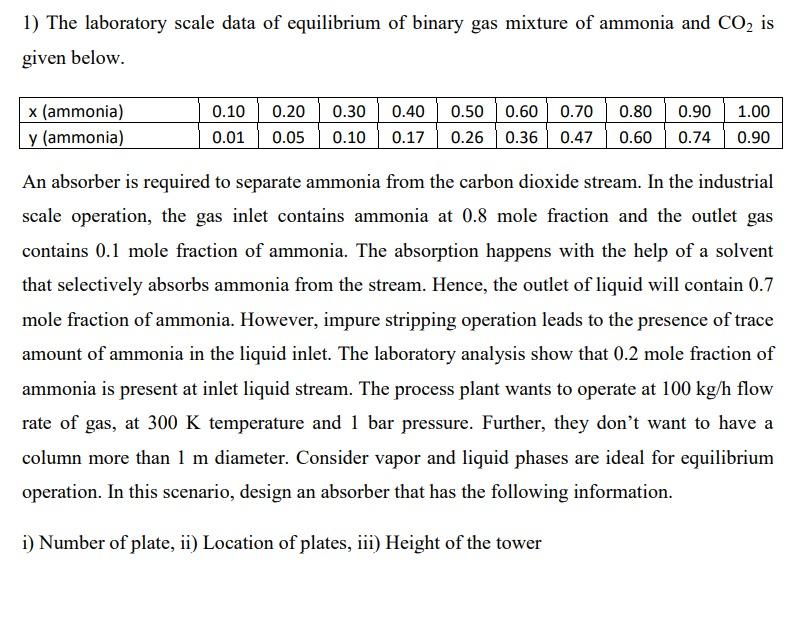
It's separation process question 1) The laboratory scale data of equilibrium of binary gas mixture of ammonia and CO2 is given below. 0.10 0.90 x
It's separation process question
1) The laboratory scale data of equilibrium of binary gas mixture of ammonia and CO2 is given below. 0.10 0.90 x (ammonia) y (ammonia) 0.20 0.05 0.30 0.10 0.40 0.17 0.50 0.60 0.70 0.80 0.26 0.36 0.47 0.60 1.00 0.90 0.01 0.74 An absorber is required to separate ammonia from the carbon dioxide stream. In the industrial scale operation, the gas inlet contains ammonia at 0.8 mole fraction and the outlet gas contains 0.1 mole fraction of ammonia. The absorption happens with the help of a solvent that selectively absorbs ammonia from the stream. Hence, the outlet of liquid will contain 0.7 mole fraction of ammonia. However, impure stripping operation leads to the presence of trace amount of ammonia in the liquid inlet. The laboratory analysis show that 0.2 mole fraction of ammonia is present at inlet liquid stream. The process plant wants to operate at 100 kg/h flow rate of gas, at 300 K temperature and 1 bar pressure. Further, they don't want to have a column more than 1 m diameter. Consider vapor and liquid phases are ideal for equilibrium operation. In this scenario, design an absorber that has the following information. i) Number of plate, ii) Location of plates, iii) Height of the towerStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


