Answered step by step
Verified Expert Solution
Question
1 Approved Answer
A 0.06-kg ice cube at -29C is placed in 0.4 kg of 39C water in a very well-insulated container. What is the final temperature

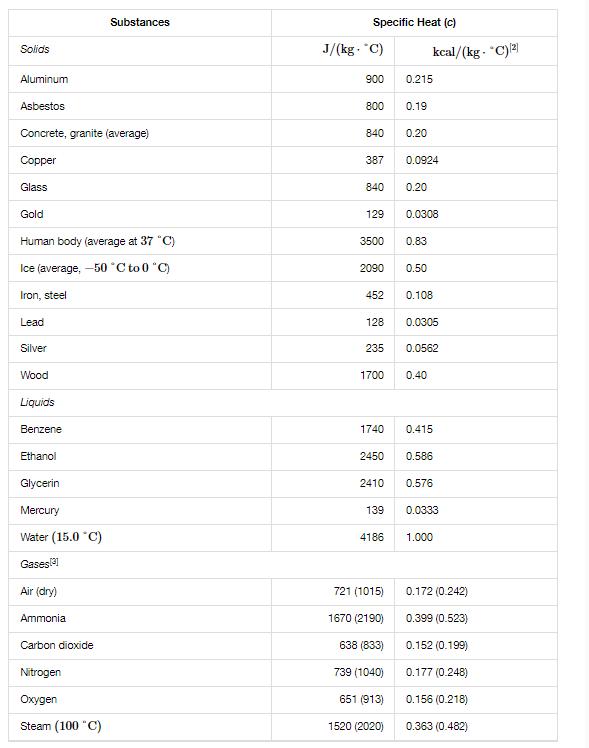
A 0.06-kg ice cube at -29C is placed in 0.4 kg of 39C water in a very well-insulated container. What is the final temperature of the mixture at equilibrium? Hint Based on given numbers, make a reasonable guess that all ice will melt. Then write down heat input into ice in three terms: (1) heat input as ice warms up to 0C, (2) heat input as ice melts into water, and (3) heat input as water from melted ice heats up to the final temperature. First two steps can be done independently of final temperature; step (3) will need to be written in terms of final temperature, If, and you will need to go through some algebra to solve for Tf, after equating heat input into ice with heat output from water. As needed, review Section 1.4 Heat Transfer, Specific Heat, and Calorimetry and Section 1.5 Phase Changes for relevant examples. The final temperature of the mixture is *C. Solids Aluminum Asbestos Concrete, granite (average) Copper Glass Gold Human body (average at 37 C) Ice (average, -50C to 0 C) Iron, steel Lead Silver Wood Liquids Benzene Ethanol Glycerin Mercury Water (15.0 C) Gases[3] Air (dry) Ammonia Substances Carbon dioxide Nitrogen Oxygen Steam (100 "C) Specific Heat (c) J/(kg. "C) 900 800 840 387 840 129 3500 2090 452 128 235 1700 1740 2450 2410 139 4186 721 (1015) 1670 (2190) 638 (833) 739 (1040) 651 (913) 1520 (2020) 0.215 0.19 0.20 0.0924 0.20 0.0308 0.83 0.50 kcal/(kg- "C)2) 0.108 0.0305 0.0562 0.40 0.415 0.586 0.576 0.0333 1.000 0.172 (0.242) 0.399 (0.523) 0.152 (0.199) 0.177 (0.248) 0.156 (0.218) 0.363 (0.482)
Step by Step Solution
★★★★★
3.51 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
To solve this problem we need to calculate the energy required for the ice to reach 0C the energy re...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


