Answered step by step
Verified Expert Solution
Question
1 Approved Answer
1. In 5 separate test tubes place 2 mL each of the following solutions: a. mercury (I) nitrate b. copper (II) nitrate c. lead

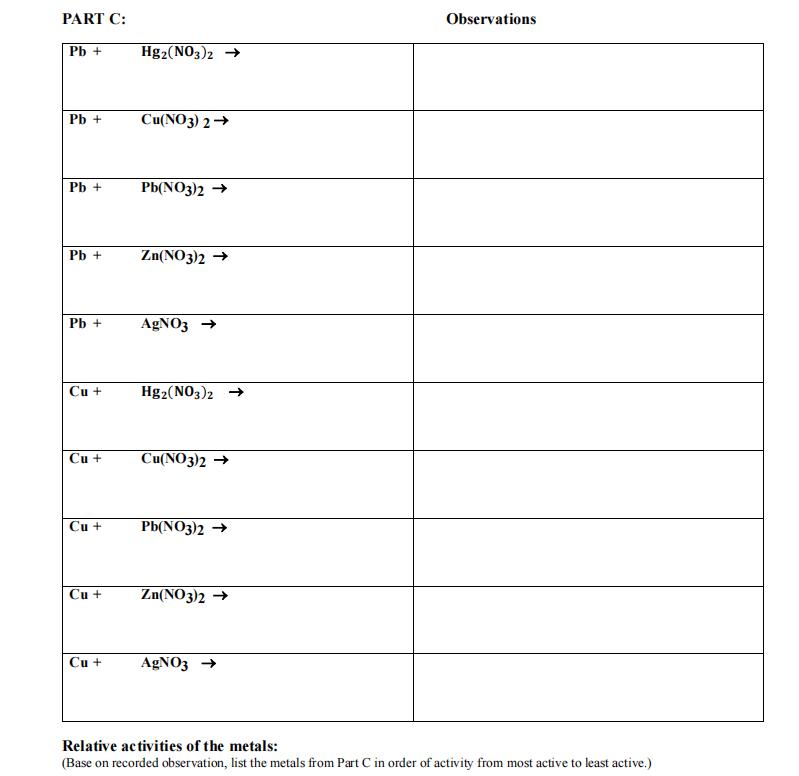
1. In 5 separate test tubes place 2 mL each of the following solutions: a. mercury (I) nitrate b. copper (II) nitrate c. lead (II) nitrate d. zinc nitrate e. silver nitrate 2. Into each of the above five test tubes place a CLEAN strip of lead metal. 3. Allow the reaction to stand for at least five minutes and observe any changes (change of color of the solution, change in the appearance of the metal, etc). Record all observations on your Report Sheet. 4. Write complete balanced chemical equations for any reactions which occur. 5. Repeat steps #1-4 using a strip of copper and fresh samples of all solutions. 6. Record all observations and write equations for the corresponding reactions. PART C: CLEAN-UP Put ALL waste into the container marked "PART C WASTE". PART C: Relative Activities Arrange the metals (Hg, Cu, Pb, Zn and Ag) in order of activity from most active to least active on your Report Sheet. PART C: Pb+ Hg2(NO3)2 Pb + Cu(NO3)2 Pb+ Pb(NO3)2 Pb + Zn(NO3)2 Pb + AgNO3 Cu + Hg2(NO3)2 Cu + Cu(NO3)2 Cu + Pb(NO3)2 Cu + Zn(NO3)2 Cu + AgNO3 Observations Relative activities of the metals: (Base on recorded observation, list the metals from Part C in order of activity from most active to least active.)
Step by Step Solution
★★★★★
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


