Answered step by step
Verified Expert Solution
Question
1 Approved Answer
just balancing the equation and question 4 Sabacie acid Sabacoyl chloride Sabacoyl chloride 1,6-hexanediamine nylon Figure 1. Chemical reaction of the synthesis of nylon. 1.
just balancing the equation and question 4 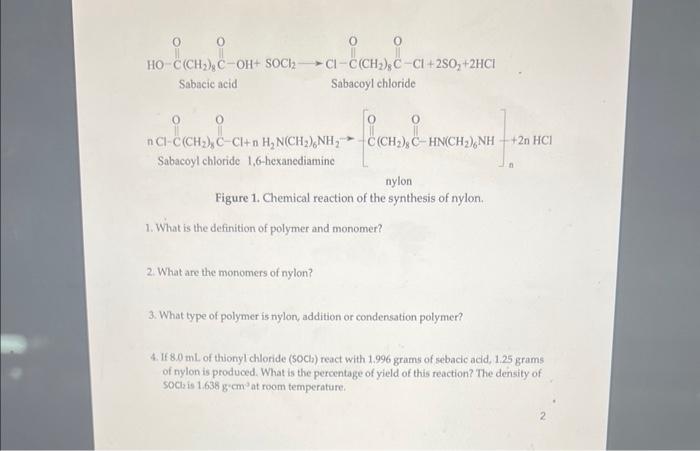
Sabacie acid Sabacoyl chloride Sabacoyl chloride 1,6-hexanediamine nylon Figure 1. Chemical reaction of the synthesis of nylon. 1. What is the definition of polymer and monomer? 2. What are the monomers of nylon? 3. What type of polymer is nylon, addition or condensation polymer? 4. If 8.0mL of thionyl chloride ( (SOCl) react with 1.996grams of sebacic acid, 1.25grams of nylon is produced. What is the percentage of yield of this reaction? The density of SOCl2 is 1.638g2cm3 at room temperature 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


