Just need help filling out missing info under "BUFFERS" section
Volumes and data needed are provided in pictures
Thanks!
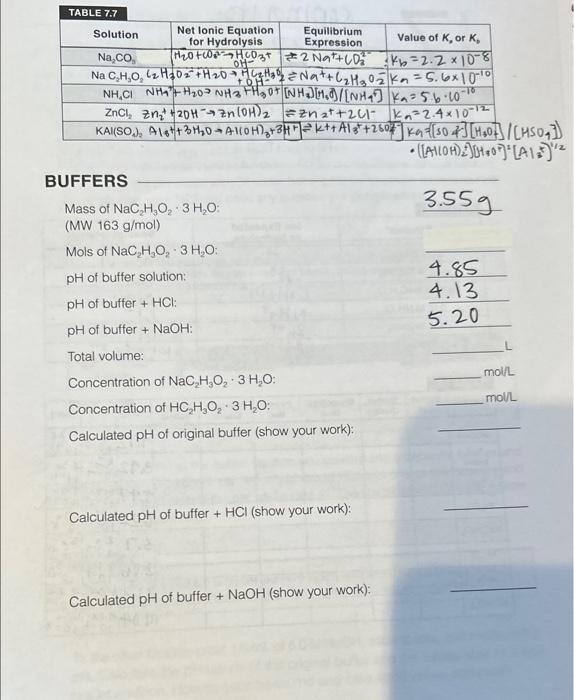
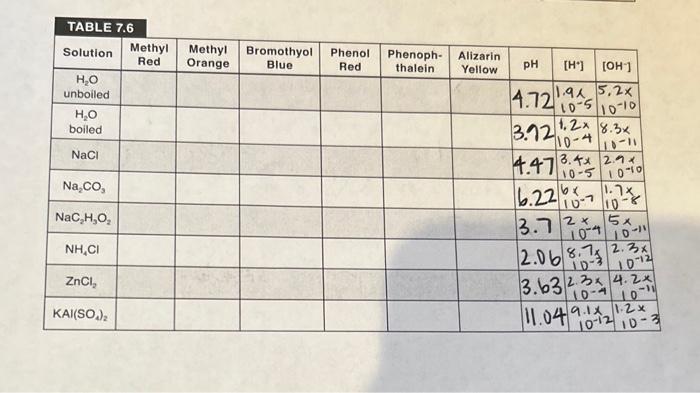
TABLE 7.7 BUFFERS Mass of NaC2H3O23H2O : 3.559 (MW 163g/mol ) Mols of NaC2H3O23H2O : pH of buffer solution: 4.85 4.13 pH of buffer +HCl : pH of buffer +NaOH : 5.20 Total volume: Concentration of NaC2H3O23H2O : mollL Concentration of HC2H3O23H2O : mol/2 Calculated pH of original buffer (show your work): Calculated pH of buffer +HCl (show your work): Calculated pH of buffer +NaOH (show your work): Boil approximately 500mL of distilled water for about 10 minutes to expel the carbon dioxide and allow the water to cool to room temperature. Add 5mL of un-boiled distilled water to each of six test tubes (one tube per indicator). Then add 3 drops of indicator and record the colors in your report sheet. From the color in these test tubes and indicator chart in lab, on the wall, determine the pH of the water. (Note: It should be acidic due to the carbon dioxide.) Empty and rinse the test tubes with boiled water and repeat this process. Allow the boiled water to completely cool to room temperature before adding the indicator, and again, use the indicator sheet on the wall to determine the pH of the boiled water (should be close to pH7 ). Repeat this same procedure to determine the pH of 0.1M solutions of NaCl, with small amounts of boiled water. From the pH you have determined, calculate the hydronium and hydroxide ion concentrations for each solution. Then complete the tables on the report sheets and calculate the Ka or Kb as appropriate. pH OF BUFFER SOLUTIONS Weigh about 3.5 grams of sodium acetate NaC2H3O23H2O to the nearest 0.01g, record the mass, and add to 150mL beaker. Measure out 8.8mL of 3.0M acetic acid and add it to the beaker of the sodium acetate. Use a graduated cylinder to measure 55.6mL of DI water and add to the solution, stirring until it dissolves. Measure the pH with our new wireless pH meters. Our new wireless pH meters will link to your cell phone and work directly with no calibration, but it is a good idea to check the pH of a known solution before relying on the pH measurement for the rest of your calculations. Always remember to rinse the pH meter with DI water between uses to obtain more accurate readings. See your TA if you have any questions or complications. CAUTION! Please be extremely careful with the bulb (tip of the pH meter), as it is made of glass and can break, and is expensivellI Pour half (32mL) of the buffer solution you created into a separate beaker and label the two beakers. Pipet 1.0mL of 6.0MHCl into one of the beakers and allow to mix and record the pH. Make sure to rinse the pipet with DI water before using for the next step. CAUTION! 6.0MHCl and 6.0MNaOH are very acidic and basic, respectively, and can cause severe chemical burns, so avoid any skin contact. To the other beaker, pipet 1.0mL of 6.0MNaOH, allow to mix, and measure the pH. Calculate the pH values of the original buffer and the values after HCl and NaOH addition. How do your measured and calculated values compare? TABLE 7.6













