just need help with calculations
the unknown solvent was malonic acid and we used .501g and i dont have the mass of the other one but it was 10.0mL of acetic acid with a density of 1.049 g/mol. i hope that helps
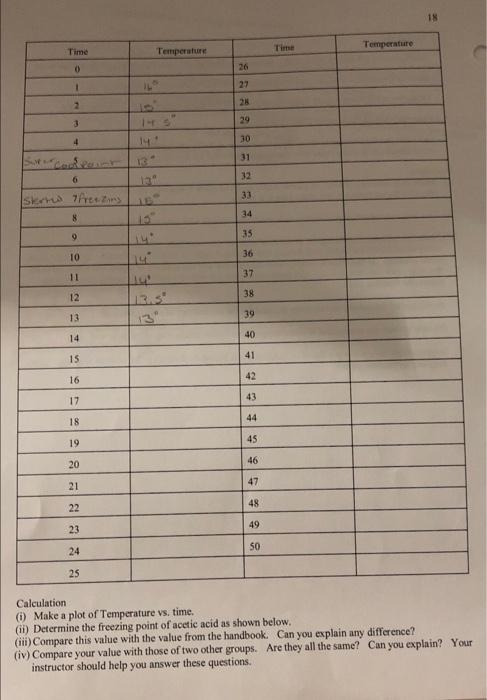
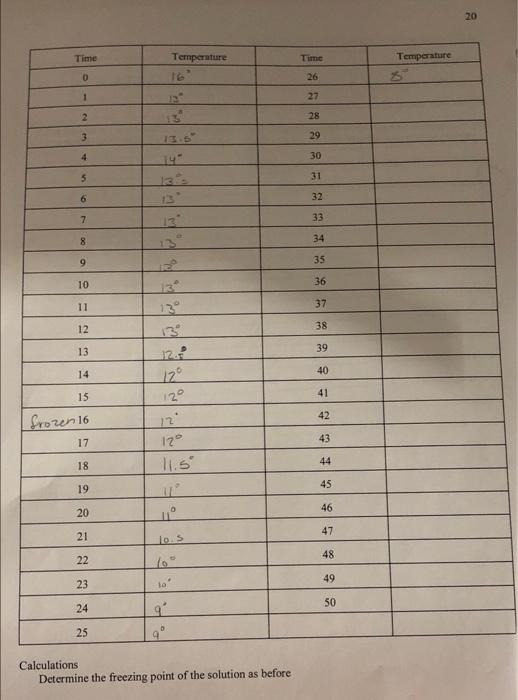
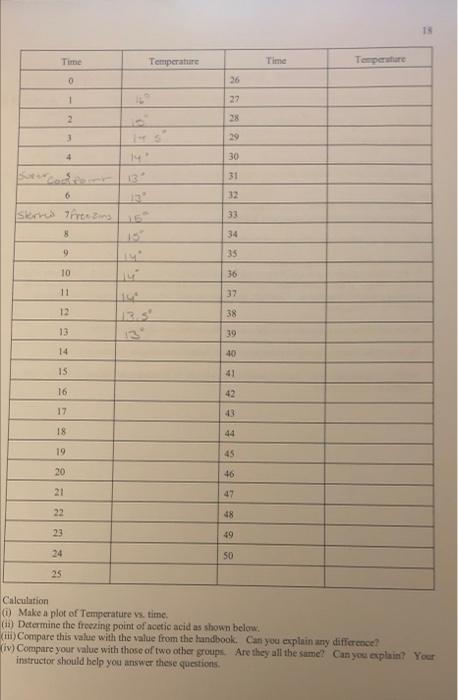
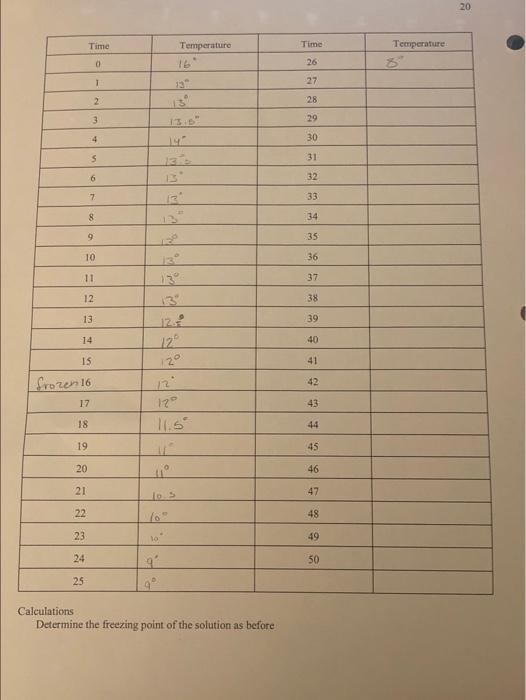
18 Time Temperature Temperature Time 0 26 1 IL 27 2 28 3 29 4 30 31 13 32 6 33 SK Tests 34 8 9 35 10 36 11 37 12 38 13.3 13 39 14 40 15 41 16 42 43 17 44 18 19 45 20 46 47 21 22 48 23 49 SO 24 25 Calculation () Make a plot of Temperature vs. time. (ii) Determine the freezing point of acetic acid as shown below. (iii) Compare this value with the value from the handbook. Can you explain any difference? (iv) Compare your value with those of two other groups. Are they all the same? Can you explain? Your instructor should help you answer these questions 20 Time Temperature Time Temperature O 26 1 12 27 2 28 3 29 4 30 5 31 6 13 32 7 33 12 8 34 9 35 10 36 11 37 12 38 13 13 39 14 40 15 41 42 frozen 16 12 12 17 43 18 44 11.5 19 45 20 46 11 47 21 los 48 22 49 23 ta' 50 24 25 9 Calculations Determine the freezing point of the solution as before 21 3.5 betermining Trand the molecular wt. AT-Teacetic acid) BT (solution) b) Using this value of AT: (positive) calculate the molecular weight of the unknown solid using the equation derived in 5.1. Damer Your instructor will give you the formula of the unknown solid from which you will calculate the theoretical value of the molecular weight of the solid. haloviec, Calculate the % error and explain any error incurred. How would you improve the experiment? Your report and calculations should be on separate sheets of paper, neatly written in pen or typed. . 13 Time Temperature Time Temperature 0 26 1 22 2 28 3 29 4 30 3 31 0 32 33 Iskred freezmo 8 34 9 35 10 36 11 37 12 13 38 13 39 13 14 40 15 41 16 42 17 43 18 44 19 45 20 46 47 22 48 23 49 34 50 25 Calculation 0 Make a plot of Temperature vs. time, (1) Determine the freezing point of acetic acid as shown below. Viti) Compare this value with the value from the handbook. Can you explain any difference? (iv) Compare your value with those of two other groups. Are they all the same? Can you explain! Your instructor should help you answer these questions 19 5.4 Determination of the freezing point of the solution. Procedure: (1) Accurately weigh 0.500g of the unknown solid on an electronic or any reliable balance. Solg (tl) Add with caution all the unknown solid to the now thawed acetic acid. (iii) Stir carefully with the thermometer and then begin temperature measurement every minute as in 5.3. Again continue measurement until the solution freezes and remain frozen for five to ten minutes. (iv) Dispose of the solution per your instructors advice. Coy ( Make a plot of temperature vs. time as before. 20 Time Temperature Temperature 16 Time 26 0 1 27 2 28 3 29 4 30 14 5 31 6 32 7 33 8 34 9 35 10 36 11 37 12 13 38 13 12 39 14 40 122 15 41 frozen 16 42 2 129 12 11.59 17 43 18 44 19 45 20 46 21 47 10 22 48 10 23 49 24 50 25 Calculations Determine the freezing point of the solution as before 5.5 Determining T, and the molecular wt. AT:-Tracetic acid) B T: (solution) b. Using this value of A Tr (positive) calculate the molecular weight of the unknown solid using the equation derived in 5.1. Borkmenuotion Your instructor will give you the formula of the unknown solid from which you will calculate the theoretical value of the molecular weight of the solid malorie acid c. Calculate the % error and explain any error incurred. How would you improve the experiment? Your report and calculations should be on separate sheets of paper, neatly written in pen or typed. 18 Time Temperature Temperature Time 0 26 1 IL 27 2 28 3 29 4 30 31 13 32 6 33 SK Tests 34 8 9 35 10 36 11 37 12 38 13.3 13 39 14 40 15 41 16 42 43 17 44 18 19 45 20 46 47 21 22 48 23 49 SO 24 25 Calculation () Make a plot of Temperature vs. time. (ii) Determine the freezing point of acetic acid as shown below. (iii) Compare this value with the value from the handbook. Can you explain any difference? (iv) Compare your value with those of two other groups. Are they all the same? Can you explain? Your instructor should help you answer these questions 20 Time Temperature Time Temperature O 26 1 12 27 2 28 3 29 4 30 5 31 6 13 32 7 33 12 8 34 9 35 10 36 11 37 12 38 13 13 39 14 40 15 41 42 frozen 16 12 12 17 43 18 44 11.5 19 45 20 46 11 47 21 los 48 22 49 23 ta' 50 24 25 9 Calculations Determine the freezing point of the solution as before 21 3.5 betermining Trand the molecular wt. AT-Teacetic acid) BT (solution) b) Using this value of AT: (positive) calculate the molecular weight of the unknown solid using the equation derived in 5.1. Damer Your instructor will give you the formula of the unknown solid from which you will calculate the theoretical value of the molecular weight of the solid. haloviec, Calculate the % error and explain any error incurred. How would you improve the experiment? Your report and calculations should be on separate sheets of paper, neatly written in pen or typed. . 13 Time Temperature Time Temperature 0 26 1 22 2 28 3 29 4 30 3 31 0 32 33 Iskred freezmo 8 34 9 35 10 36 11 37 12 13 38 13 39 13 14 40 15 41 16 42 17 43 18 44 19 45 20 46 47 22 48 23 49 34 50 25 Calculation 0 Make a plot of Temperature vs. time, (1) Determine the freezing point of acetic acid as shown below. Viti) Compare this value with the value from the handbook. Can you explain any difference? (iv) Compare your value with those of two other groups. Are they all the same? Can you explain! Your instructor should help you answer these questions 19 5.4 Determination of the freezing point of the solution. Procedure: (1) Accurately weigh 0.500g of the unknown solid on an electronic or any reliable balance. Solg (tl) Add with caution all the unknown solid to the now thawed acetic acid. (iii) Stir carefully with the thermometer and then begin temperature measurement every minute as in 5.3. Again continue measurement until the solution freezes and remain frozen for five to ten minutes. (iv) Dispose of the solution per your instructors advice. Coy ( Make a plot of temperature vs. time as before. 20 Time Temperature Temperature 16 Time 26 0 1 27 2 28 3 29 4 30 14 5 31 6 32 7 33 8 34 9 35 10 36 11 37 12 13 38 13 12 39 14 40 122 15 41 frozen 16 42 2 129 12 11.59 17 43 18 44 19 45 20 46 21 47 10 22 48 10 23 49 24 50 25 Calculations Determine the freezing point of the solution as before 5.5 Determining T, and the molecular wt. AT:-Tracetic acid) B T: (solution) b. Using this value of A Tr (positive) calculate the molecular weight of the unknown solid using the equation derived in 5.1. Borkmenuotion Your instructor will give you the formula of the unknown solid from which you will calculate the theoretical value of the molecular weight of the solid malorie acid c. Calculate the % error and explain any error incurred. How would you improve the experiment? Your report and calculations should be on separate sheets of paper, neatly written in pen or typed