Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Just need help with sample 1 I can do Sample 2 Also for 6a is asking if its either O or Cu Mass of copper
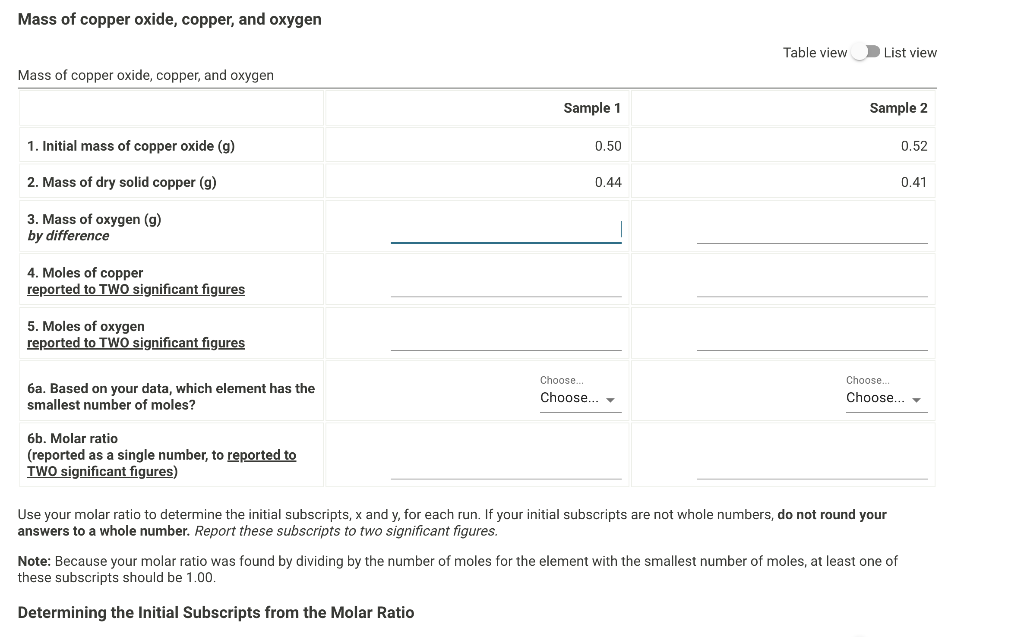
Just need help with sample 1 I can do Sample 2
Also for 6a is asking if its either O or Cu
Mass of copper oxide, copper, and oxygen Table view List view Mass of copper oxide. copper. and oxvaen Use your molar ratio to determine the initial subscripts, x and y, for each run. If your initial subscripts are not whole numbers, do not round your answers to a whole number. Report these subscripts to two significant figures. Note: Because your molar ratio was found by dividing by the number of moles for the element with the smallest number of moles, at least one of these subscripts should be 1.00. Determining the Initial Subscripts from the Molar RatioStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


