Question
A mile is 5280 feet, a foot is 12 inches, and an inch is 0.0254 meters. Calculate how many miles are in 1 km.
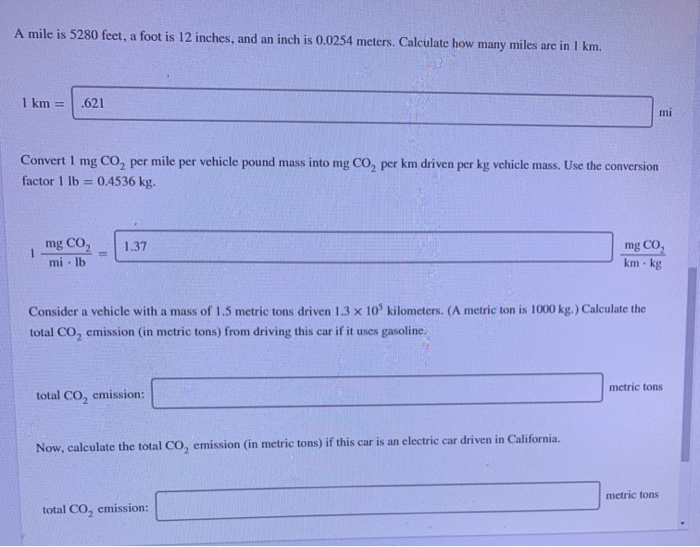

A mile is 5280 feet, a foot is 12 inches, and an inch is 0.0254 meters. Calculate how many miles are in 1 km. 1 km = .621 Convert 1 mg CO per mile per vehicle pound mass into mg CO per km driven per kg vehicle mass. Use the conversion factor 1 lb = 0.4536 kg. 1 mg CO mi lb 1.37 Consider a vehicle with a mass of 1.5 metric tons driven 1.3 x 10 kilometers. (A metric ton is 1000 kg.) Calculate the total CO emission (in metric tons) from driving this car if it uses gasoline. total CO emission: Now, calculate the total CO emission (in metric tons) if this car is an electric car driven in California. total CO emission: mg CO km-kg mi metric tons metric tons Convert 1 mg CO per mile per vehicle pound mass into mg CO per km driven per kg vehicle mass. Use the conversion factor 1 lb = 0.4536 kg. 1 mg CO mi-lb 1.37 Consider a vehicle with a mass of 1.5 metric tons driven 1.3 x 10 kilometers. (A metric ton is 1000 kg.) Calculate the total CO emission (in metric tons) from driving this car if it uses gasoline. total CO emission: Now, calculate the total CO emission (in metric tons) if this car is an electric car driven in California. total CO emission: Which car emits more CO? This comparison does not include CO emitted in manufacturing the car. mg CO km - kg the electric car the gasoline car metric tons metric tons
Step by Step Solution
3.40 Rating (172 Votes )
There are 3 Steps involved in it
Step: 1
Question 1 To calculate how many miles in 1 kilometer we can use the following conversions 1 mile 5280 feet 1 foot 12 inches 1 inch 00254 meter now le...
Get Instant Access with AI-Powered Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


