Answered step by step
Verified Expert Solution
Question
1 Approved Answer
^^ just the third part with the final equilibrium concentrations please! I've used the quadratic formula but I think I'm doing the formula incorrectly The
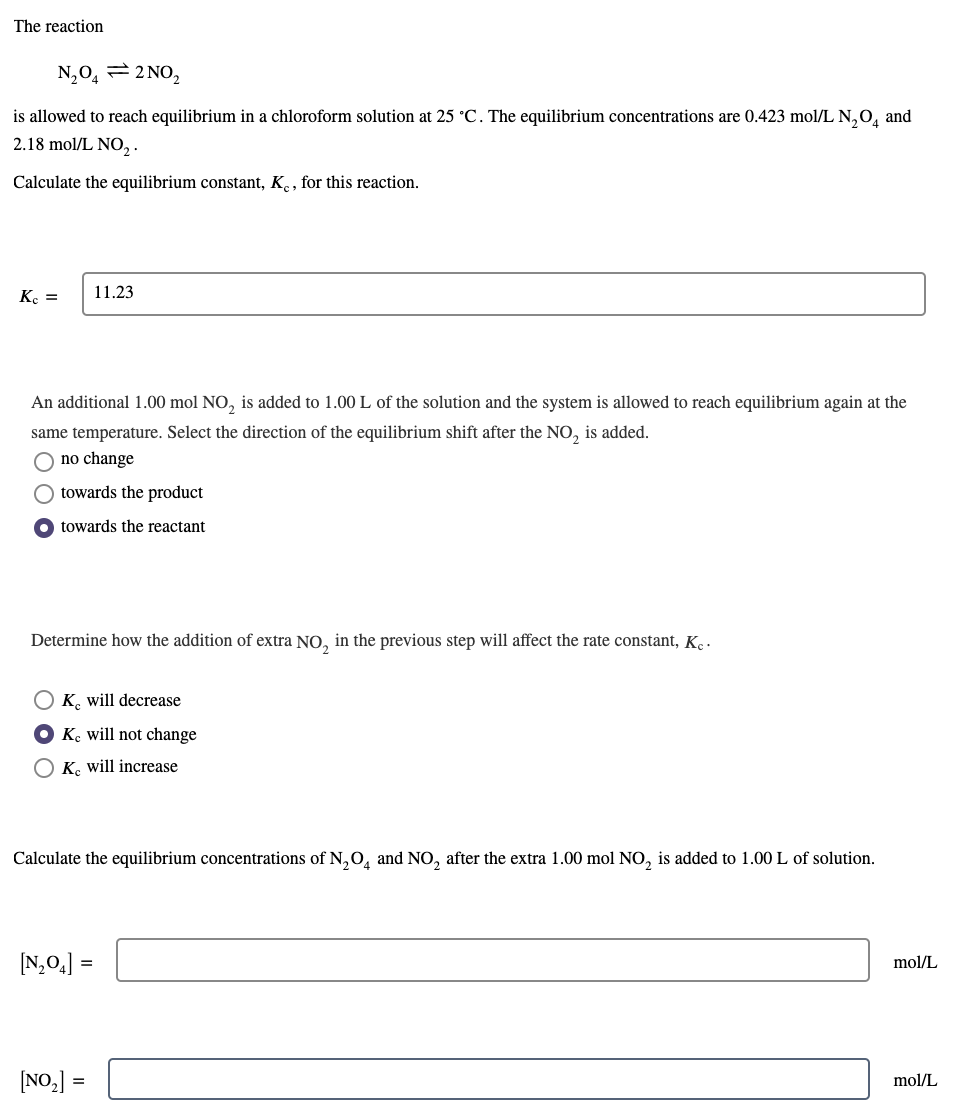
^^ just the third part with the final equilibrium concentrations please! I've used the quadratic formula but I think I'm doing the formula incorrectly
The reaction N204 = 2 NO, is allowed to reach equilibrium in a chloroform solution at 25 C. The equilibrium concentrations are 0.423 mol/L N204 and 2.18 mol/L NO, Calculate the equilibrium constant, K, for this reaction. K. = 11.23 An additional 1.00 mol NO, is added to 1.00 L of the solution and the system is allowed to reach equilibrium again at the same temperature. Select the direction of the equilibrium shift after the NO, is added. O no change towards the product O towards the reactant Determine how the addition of extra NO, in the previous step will affect the rate constant, Kc. OK. will decrease Ke will not change OK will increase Calculate the equilibrium concentrations of N2O4 and NO, after the extra 1.00 mol NO, is added to 1.00 L of solution. (N204] = mol/L [NO2] = mol/LStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


