Answered step by step
Verified Expert Solution
Question
1 Approved Answer
K* Complete the following table regarding the naming of polyatomic ionic compounds cation anion Chemical formula NH NH41 NH NHASDA CO(PO4)2 Al3+ Al+ Ca+
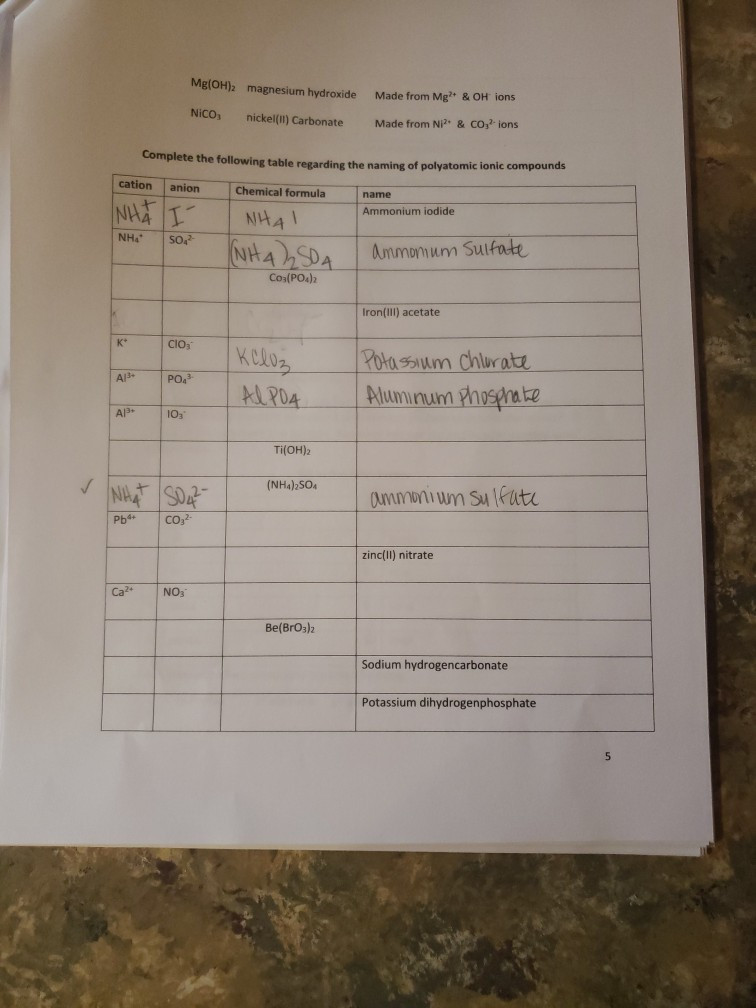
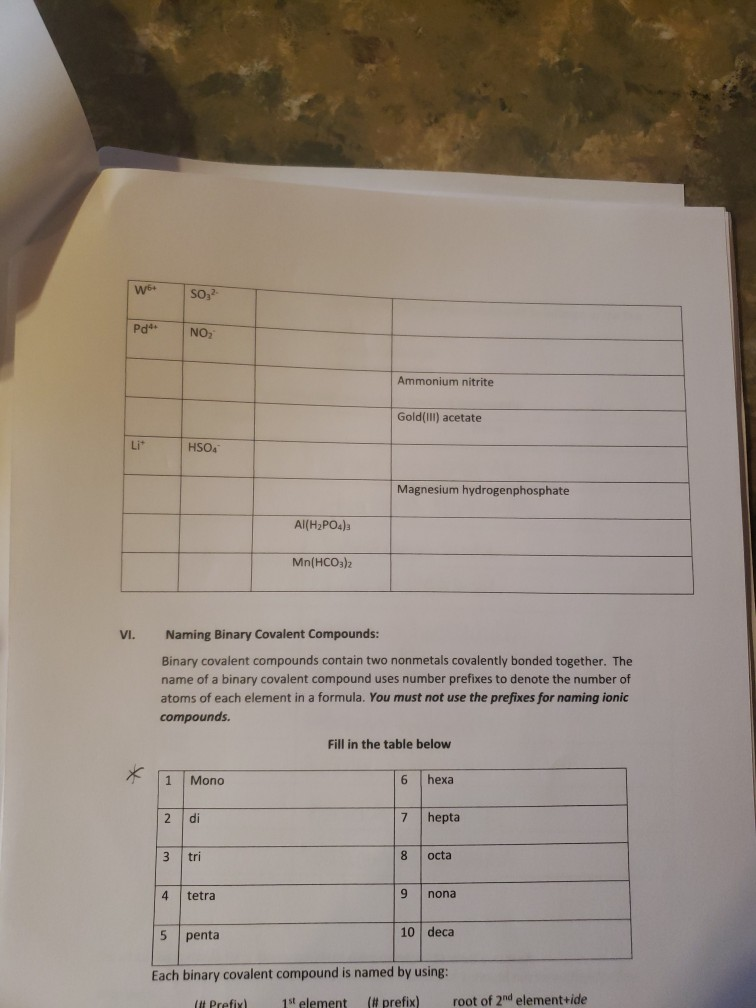

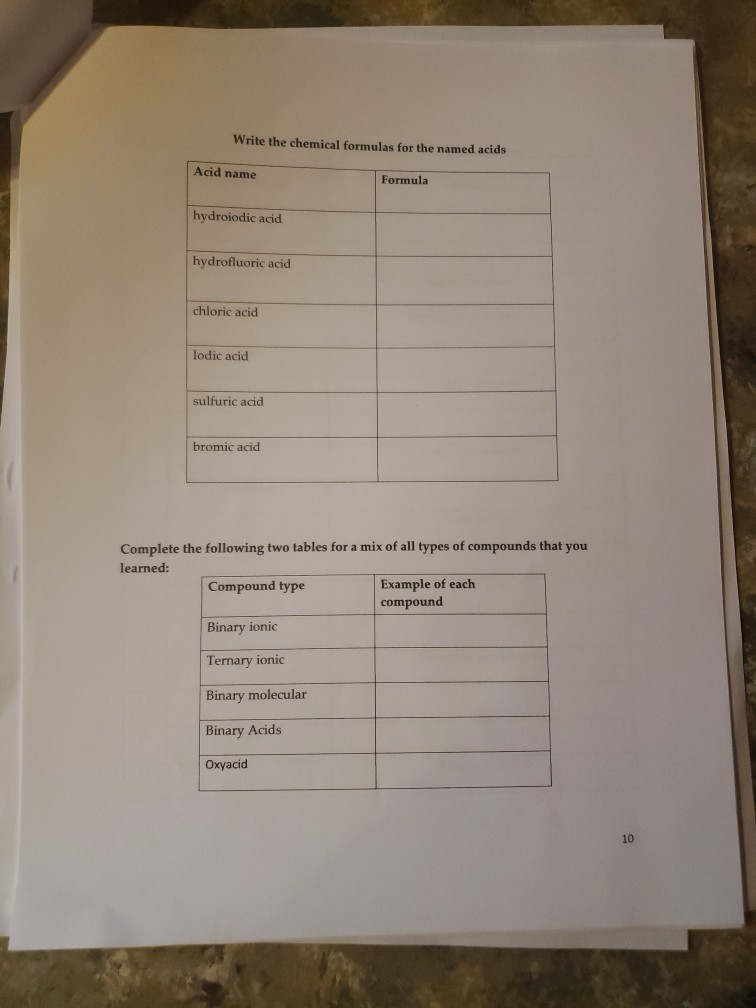
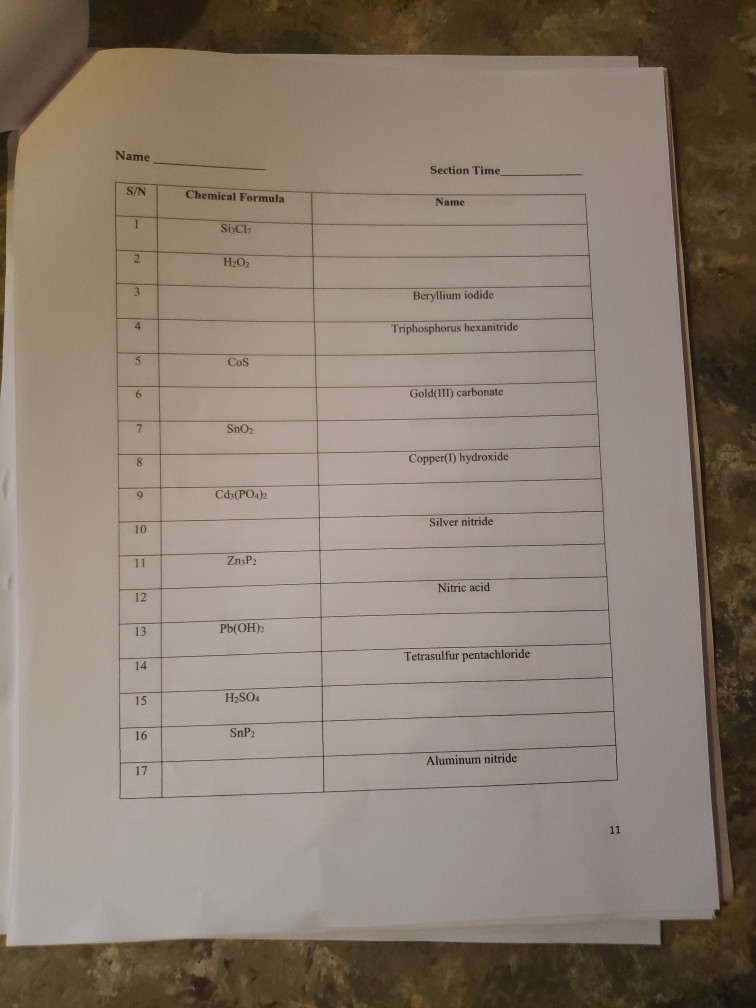
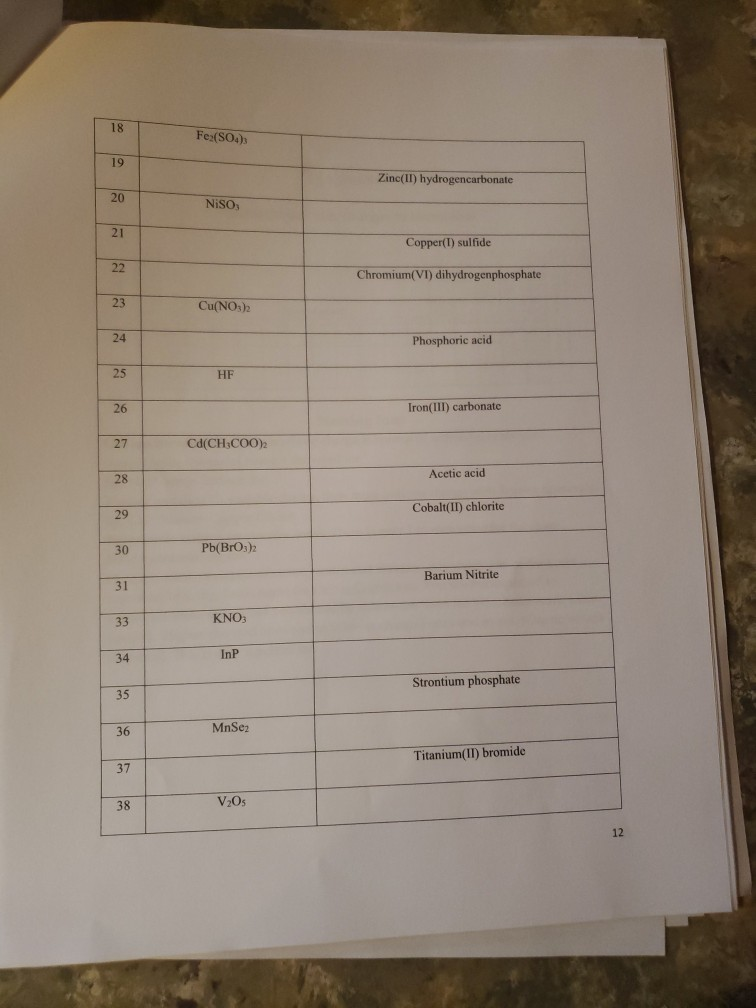
K* Complete the following table regarding the naming of polyatomic ionic compounds cation anion Chemical formula NH NH41 NH NHASDA CO(PO4)2 Al3+ Al+ Ca+ Mg(OH)2 magnesium hydroxide NICO nickel(II) Carbonate SO4- CIOs PO4 10 NH SO Pb4+ CO32- NO AlPO4 Ti(OH)2 (NH4)2SO4 Made from Mg2+ & OH ions Made from Ni+ & CO3- ions Be(BrO3)2 name Ammonium iodide Ammomum Sulfate Iron(III) acetate Potassium Chlorate Aluminum Phosphate ammonium sulfate zinc(II) nitrate Sodium hydrogencarbonate Potassium dihydrogenphosphate W5+ Pd4 Li VI. SO- 1 NO HSOA Mono 2 di 3 tri Naming Binary Covalent Compounds: Binary covalent compounds contain two nonmetals covalently bonded together. The name of a binary covalent compound uses number prefixes to denote the number of atoms of each element in a formula. You must not use the prefixes for naming ionic compounds. 4 tetra Al(HPO4)3 5 penta Mn(HCO3)2 Ammonium nitrite Gold(III) acetate Magnesium hydrogenphosphate Fill in the table below 6 7 8 9 hexa hepta octa nona 10 deca Each binary covalent compound is named by using: ( Prefix) 1st element (# prefix) root of 2nd element+ide Write a formula for the following binary covalent compounds: carbon disulfide sulfur trioxide dinitrogen pentoxide trisilicon tetrahydride sulfur hexafluoride Trisulfur heptanitride VII. Naming molecular acids: Acids are uncharged molecules. They contain the familiar anions with added H* ions. Naming Binary Acids: Binary acids (two atoms types only, hydrogen + a nonmetal) are named by adding hydro at the beginning of the respective anion name and the end of the anion name is changed to ic acid from ide. Ex. HCl, a binary acid is named: hydrochloric acid (respective anion is chloride [Cl]). Naming ternary oxyacids: Oxyacids are ternary (3 elements). They contain oxygen, hydrogen and another element. If the anion is an oxyanion then change the ate ending of the polyatomic anion that they contain to ic acid. A prefix is not needed. Most molecular ions that you have learnt about have an oxygen in them and they are therefore oxyanions. Ex. HNO3 is named: Nitric acid (respective anion is nitrate [NO3]). Continue to next page Write the chemical formulas for the named acids Acid name hydroiodic acid hydrofluoric acid chloric acid Iodic acid sulfuric acid bromic acid Complete the following two tables for a mix of all types of compounds that you learned: Compound type Formula Binary ionic Ternary ionic Binary molecular Binary Acids Oxyacid Example of each compound 10 Name S/N 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 Chemical Formula SinCh HO COS SnO Cds(PO4)2 Zn3P Pb(OH)2 HSO4 SnP Section Time_ Name Beryllium iodide Triphosphorus hexanitride Gold(III) carbonate Copper (1) hydroxide Silver nitride Nitric acid Tetrasulfur pentachloride Aluminum nitride 11 18 19 20 21 22 23 24 25 26 27 28 29 30 31 33 34 35 36 37 38 Fe (SO4)3 NISO, Cu(NO3)2 Cd(CH3COO)2 Pb(BrO3)2 KNO InP MnSe V05 Zinc(11) hydrogencarbonate Copper (1) sulfide Chromium(VI) dihydrogenphosphate Phosphoric acid Iron(III) carbonate Acetic acid Cobalt(II) chlorite Barium Nitrite Strontium phosphate Titanium(II) bromide 12
Step by Step Solution
★★★★★
3.32 Rating (140 Votes )
There are 3 Steps involved in it
Step: 1
Cation Anion Chemical Formulae Name NH4 I NH4I Ammoniu...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


