Answered step by step
Verified Expert Solution
Question
1 Approved Answer
K constant is 0.54. Please show all steps in a clear and concise manner and give right answer. Use the rate law you have formulated
K constant is 0.54. Please show all steps in a clear and concise manner and give right answer. 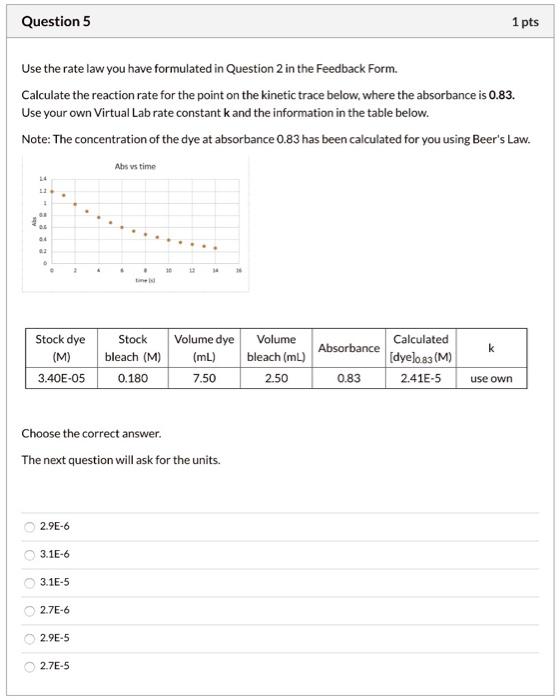
Use the rate law you have formulated in Question 2 in the Feedback Form. Calculate the reaction rate for the point on the kinetic trace below, where the absorbance is 0.83. Use your own Virtual Lab rate constant k and the information in the table below. Note: The concentration of the dye at absorbance 0.83 has been calculated for you using Beer's Law. Choose the correct answer. The next question will ask for the units. 2.9E6 3.1E6 3.1E5 2.7E6 2.9E5 2.7E5 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


