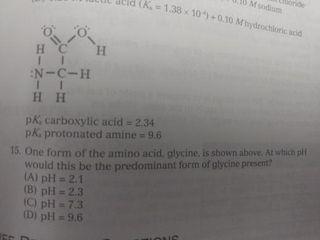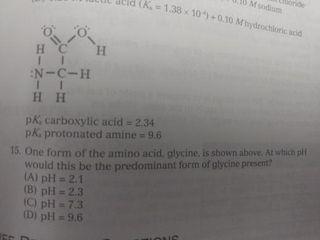

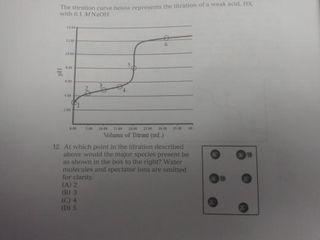

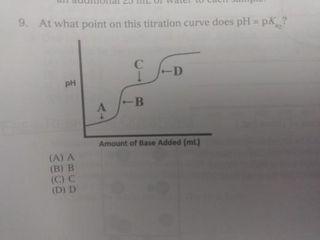
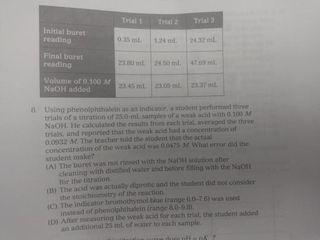




K=1.38100.10 Mydlo 0 :0 00 IT EN-C-H 11 HH pk, carboxylic acid = 2.34 pk, protonated amine 9.6 15. One form of the amino acid glycine is shown above. At which would this be the predominant form of glycine present Al pH = 2.1 (B) pH = 23 HC pH13 D) pH 6 13. At which point in the titration described above would the major species present be as shown in the box to the right? Wister molecules and spectator ions are omitted for darity CA3 (B) 4 (C) 5 D G 14. A researcher needs to prepare a pH 5 buffer What solutions would be best A) 0.20 Mboro acid-511 10 90/10 Msodium'borate YB 20 Mummon (-18 10.0.10 Mammonium chloride ICI 0.20 M propanole alt=1310) +0.10 M Sodium hydroxide (DO 20 Article didik - 1.38.100.10 hydrochloric acid with WNER 1. A which in species se Wit 10. A buffer can maintain relatively constant pH upon addition of small amounts of a strong base because (A) only large amounts of base will cause an appreciable pll change in an aqueous solution (B) the strong base is balanced by the weak acid and conjugate buse in the buffer (C) the weak acid reacts with the strong base to make additional conjugate base (D) only the addition of an acid would change the pH of a buffer system 11. A solution is prepared that is 0.26 Min HX and 0:10 Min X The ki of HX is 4.0.X 10 What is the pH of the solution? TA) 2.00 (B) 3,00 (C) 4.00 (D) 5,00 9. At what point on this titration curve does pH? co -D ph B A Amount of Base Added (ml) () (B) B D D Initial buret 015 Final reading tam to Volume of 0.100 ML NOH Using phenolphth trials of station mit NORH other and pred that on the heat concentrathew What the student me LAT The bus we will Then dorpheniramo SA 7A buffer with pH of 7.5 is needed. Which combination of concentrations of NaH PO. (6.210) and Na HPO, (= 4.8x 10 would give a pH closest to 7.52 INOB PO OLM IN:HPOI 0.9 M (Al 1.0 M (B) (C) 1.0 M 0.20 M 0.10 M 0.90 M 0.10 M 6: Two unlabeled Erlenmeyer flasks each contained 2500 ml. or solution at pH 4.00. Each flask was titrated with the same standardized NaOH solution. The volumes needed to reach the endpoint are listed in the table below. What claim can be made about the acids in Flasks A and B? Flask Titrant Volume (ml) 11.29 37.07 B (A) The concentration of the acids was the same because both solutions were pH 4.00. (B) The strength of the acids was the same because both solutions were pH 4.00 (C) Flask A was a strong acid because less titrant was needed to reach the endpoint (D) Flask B was a strong acid because more titrant was needed to reach the endpoint. A solution may have lost maintained in the base region through the use of a NAHON.CO, bude system. Wilchestions how this butter systum Would realistit changes www strong base is added FA) OH + HCO HO.COM (3) 01 | | | | | | | | | (CH 2 OH+H.CO2H000 D) 5. 0.0040 mole HC was added to a litration of 100 ml, of 0.10 ANH solution. The resulting solution was thoroughly mixed. Assume that volume does not change it the species in order of decreasing equilibrium concentration at this point in the titration (A) INHINHNHOH B) INHA > INHI TOHIHI ) (OHH ||NH - N1, H| D) NHL > INHA HITORI Questions 1 and 2 use the following information Acid Acetic acid Ben olc acid Hydrofluoric acid Lactic acid ka 1810 5410 72. 10- 1410 1. Which of the selds in the table above along with their conjugate base, would make the most effective buffer to maintain the pH of a solution 4852 Al Acetic acid (B) Benzoic acid c) Hydrofluoric acid Dy lactic acid