Answered step by step
Verified Expert Solution
Question
1 Approved Answer
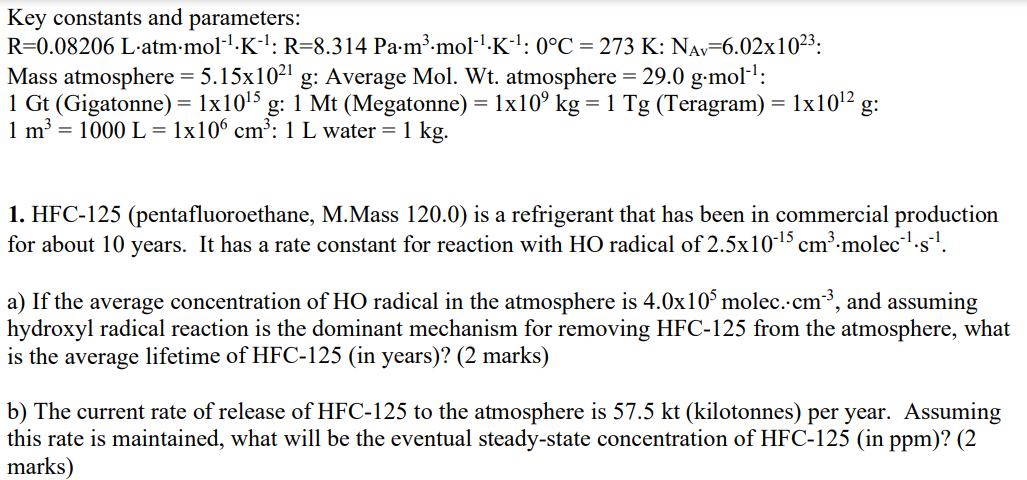
Key constants and parameters: R=0.08206Latmmol1K1:R=8.314Pam3mol1K1:0C=273K:NAv=6.021023: Mass atmosphere =5.151021g : Average Mol. Wt. atmosphere =29.0gmol1 : 1Gt (Gigatonne) =11015g:1Mt (Megatonne) =1109kg=1Tg( Teragram )=11012g : 1m3=1000L=1106cm3:1L water
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


