Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Kindly answer this question in great detail. Consider the combination of a continuous steady state catalytic reactor followed by distillation with recycle of the unreacted
Kindly answer this question in great detail. 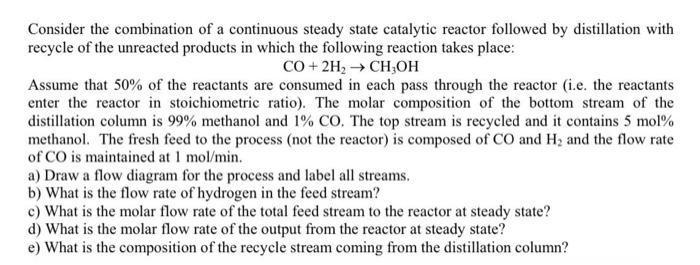
Consider the combination of a continuous steady state catalytic reactor followed by distillation with recycle of the unreacted products in which the following reaction takes place: CO+2H2CH3OH Assume that 50% of the reactants are consumed in each pass through the reactor (i.e. the reactants enter the reactor in stoichiometric ratio). The molar composition of the bottom stream of the distillation column is 99% methanol and 1%CO. The top stream is recycled and it contains 5mol% methanol. The fresh feed to the process (not the reactor) is composed of CO and H2 and the flow rate of CO is maintained at 1mol/min. a) Draw a flow diagram for the process and label all streams. b) What is the flow rate of hydrogen in the feed stream? c) What is the molar flow rate of the total feed stream to the reactor at steady state? d) What is the molar flow rate of the output from the reactor at steady state? e) What is the composition of the recycle stream coming from the distillation column 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


