KINETICS LABORATORY REPORT
I posted the entire lab so you can take a look at the data.
ANSWER THE BLANK SPACE IN THE LAB REPORT
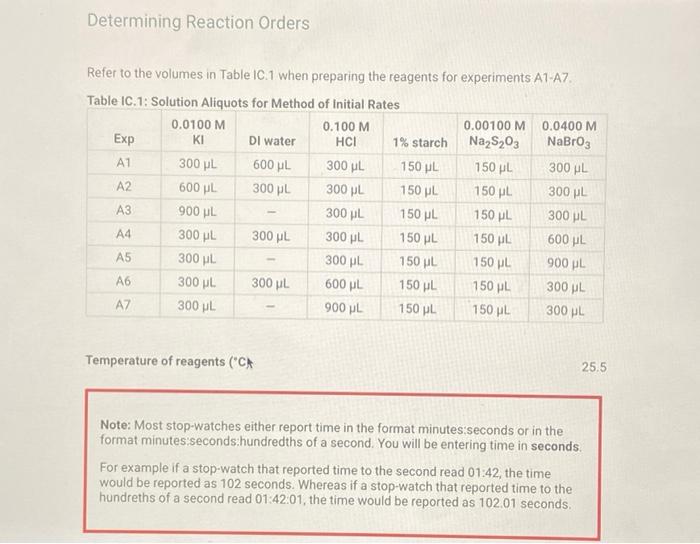
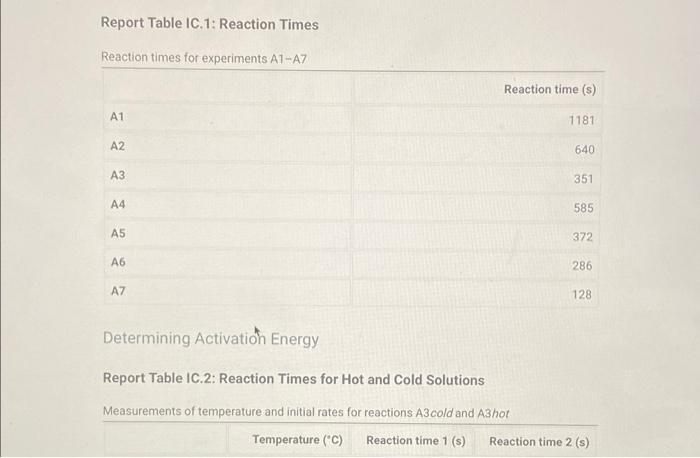
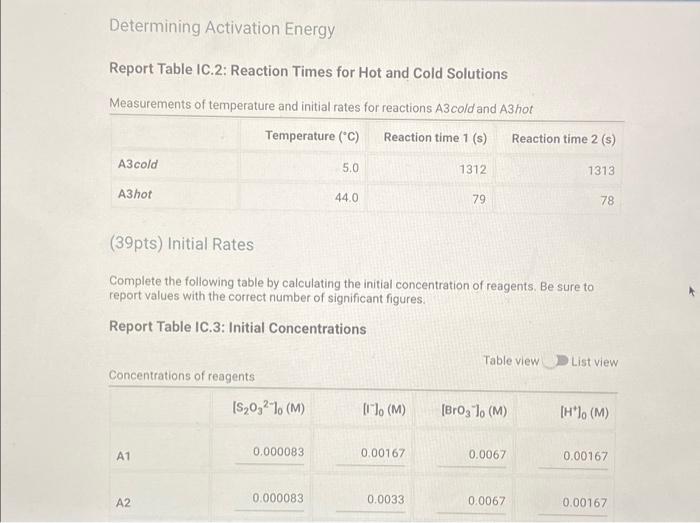
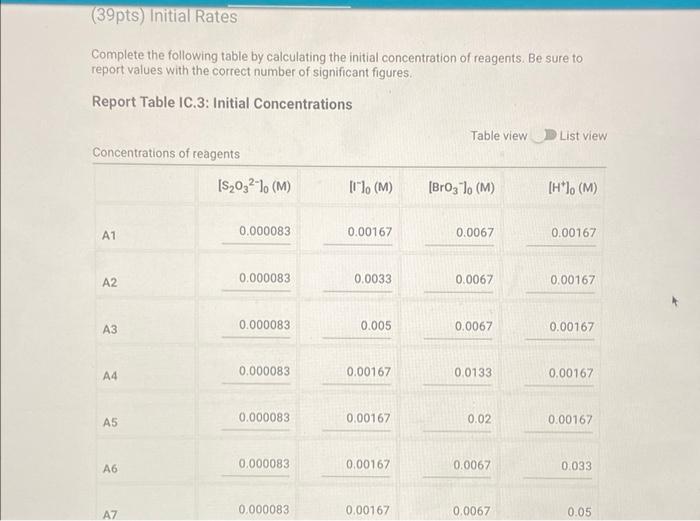
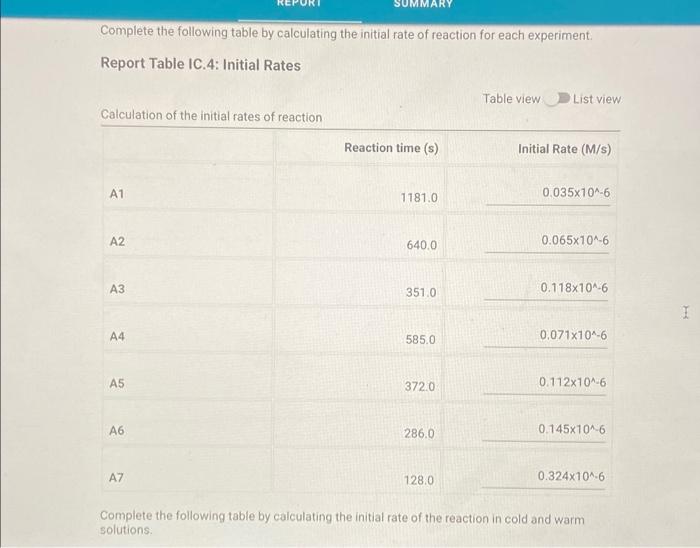
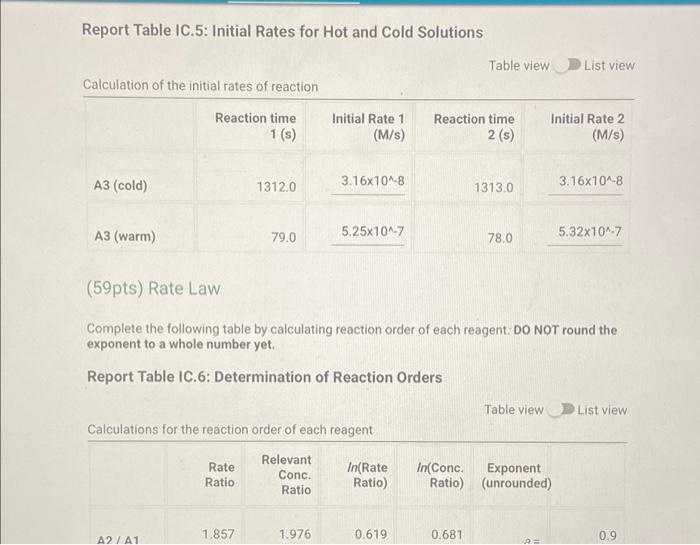
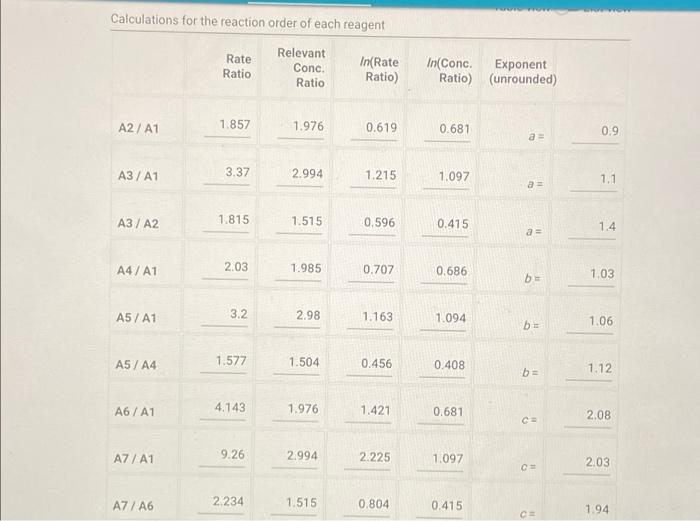
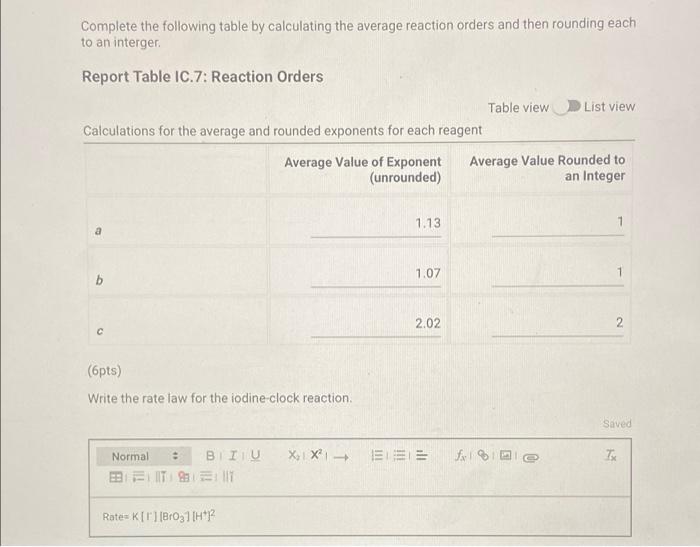
Determining Reaction Orders Refer to the volumes in Table IC.1 when preparing the reagents for experiments A1-A7. Table IC.1: Solution Aliquots for Method of Initial Rates 0.00100 M 0.0400 M 0.0100 M KI 0.100 M HCI 1% starch DI water NaS03 NaBrO3 Exp A1 300 L 600 L 300 L 150 L 300 L 150 L A2 600 L 300 L 300 L 300 L 150 L 150 L A3 900 L 300 L 150 L 300 L 150 L A4 300 L 300 L 300 L 600 L 150 L 150 L A5 300 L 300 L 150 L 150 pl 900 L A6 300 L 300 L 600 L 150 L 150 L 300 L A7 300 L 900 L 150 L 150 L 300 L 25.5 Temperature of reagents ("CA Note: Most stop-watches either report time in the format minutes:seconds or in the format minutes:seconds:hundredths of a second. You will be entering time in seconds. For example if a stop-watch that reported time to the second read 01:42, the time would be reported as 102 seconds. Whereas if a stop-watch that reported time to the hundreths of a second read 01:42:01, the time would be reported as 102.01 seconds. Report Table IC.1: Reaction Times Reaction times for experiments A1-A7 A1 A2 A3 A4 A5 A6 A7 Determining Activation Energy Report Table IC.2: Reaction Times for Hot and Cold Solutions Measurements of temperature and initial rates for reactions A3 cold and A3 hot Temperature (C) Reaction time (s) 1181 640 351 585 372 286 128 Reaction time 1 (s) Reaction time 2 (s) Determining Activation Energy Report Table IC.2: Reaction Times for Hot and Cold Solutions Measurements of temperature and initial rates for reactions A3 cold and A3 hot Temperature (C) Reaction time 1 (s) Reaction time 2 (s) A3 cold 5.0 1312 1313 A3hot 44.0 79 78 (39pts) Initial Rates Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.3: Initial Concentrations Table view List view Concentrations of reagents [S032-10 (M) 0.000083 A1 0.000083 A2 [1] (M) 0.00167 0.0033 [BrO3 lo (M) 0.0067 0.0067 [H*] (M) 0.00167 0.00167 (39pts) Initial Rates Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.3: Initial Concentrations Table view List view Concentrations of reagents [S03-10 (M) 0.000083 A1 0.000083 A2 0.000083 A3 0.000083 A4 0.000083 A5 0.000083 A6 0.000083 A7 [1] (M) 0.00167 0.0033 0.005 0.00167 0.00167 0.00167 0.00167 [BrO3 10 (M) 0.0067 0.0067 0.0067 0.0133 0.02 0.0067 0.0067 [H*] (M) 0.00167 0.00167 0.00167 0.00167 0.00167 0.033 0.05 SUMMARY Complete the following table by calculating the initial rate of reaction for each experiment. Report Table IC.4: Initial Rates Table view List view Calculation of the initial rates of reaction Reaction time (s) Initial Rate (M/s) A1 0.035x10^-6 1181.0 A2 0.065x10^-6 640.0 A3 0.118x10^-6 351.0 A4 0.071x10^-6 585.0 A5 0.112x10^-6 372.0 A6 0.145x10^-6 286.0 A7 0.324x10^-6 128.0 Complete the following table by calculating the initial rate of the reaction in cold and warm solutions. Report Table IC.5: Initial Rates for Hot and Cold Solutions Calculation of the initial rates of reaction Reaction time 1 (s) Initial Rate 1 (M/s) Reaction time 2 (s) Initial Rate 2 (M/s) A3 (cold) 3.16x10^-8 3.16x10^-8 1312.0 1313.0 A3 (warm) 5.25x10^-7 5.32x10^-7 79.0 78.0 (59pts) Rate Law Complete the following table by calculating reaction order of each reagent. DO NOT round the exponent to a whole number yet. Report Table IC.6: Determination of Reaction Orders Table view List view Calculations for the reaction order of each reagent Relevant Rate In(Rate In(Conc. Conc. Exponent Ratio) (unrounded) Ratio Ratio) Ratio 1.857 1.976 0.619 0.681 0.9 A2/A1 A= Table view List view Calculations for the reaction order of each reagent Relevant Rate In(Rate Conc. Ratio Ratio) Ratio 1.857 A2/A1 1.976 0.619 A3/A1 3.37 2.994 1.215 1.815. 1.515 A3/A2 0.596 2.03 A4/A1 1.985 0.707 3.2 2.98 A5/A1 1.163 A5/A4 1.577 1.504 0.456 4.143 1.976 1.421 A6/A1 9.26 A7/A1 2.994 2.225 2,234 1.515 0.804 A7/A6 PERDIC FO In(Conc. Exponent Ratio) (unrounded) 0.681 a= 1.097 a= 0.415 0.686 1.094 0.408 0.681 1.097 0.415 a= b= b= b= 3 c= C= 0.9 1.1 1.4 1.03 1.06 1.12 2.08 2.03 1.94 Complete the following table by calculating the average reaction orders and then rounding each to an interger. Report Table IC.7: Reaction Orders Table view List view Calculations for the average and rounded exponents for each reagent Average Value of Exponent (unrounded) Average Value Rounded to an Integer 1.13 1.07 2.02 C (6pts) Write the rate law for the iodine-clock reaction. Saved Normal BIU XX EEE fx 8 Tx BE = Rate= K[I][BrO3] [H* (11pts) Rate Constant Complete the following table by calculating the rate constant for each experiment at room temperature. Report Table IC.8: Rate Constants at Room Temperature Table view List view Calculation of the rate constants at room temperature Rate Constant A1 A2 A3 A4 A5 A6 A7 A7 Complete the following table by calculating the rate constant for the warm and cold A3 solutions Report Table IC.9: Rate Constants at Warm and Cold Temperatures Table view List view Calculation of the rate constants at different temperatures Rate Constant 1 Rate Constant 2 A3 (cold) A3 (warm) (5pts) Determination of the Activation Energy Be sure to report values with the correct number of significant figures. (1pts) Average value of rate constant k = (exclude A3 cold and A3 warm values) (1pts) Average rate constant in cold water: k = (5pts) Determination of the Activation Energy Be sure to report values with the correct number of significant figures. (1pts) Average value of rate constant: k = (exclude A3 cold and A3 warm values) (1pts) Average rate constant in cold water: k = (1pts) Average rate constant in warm water: k = Temperature of reagents at room temperature: Temperature of ice water bath: Temperature of warm water bath: (1pts) (1pts) Slope from Arrhenius plot: m = Activation Energy: E = 25.5 C 5.0 C 44.0 C