Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Knowing the relative energy levels of the atomic orbitals of carbon and nitrogen, we can construct an atomic energy level diagram such as that shown
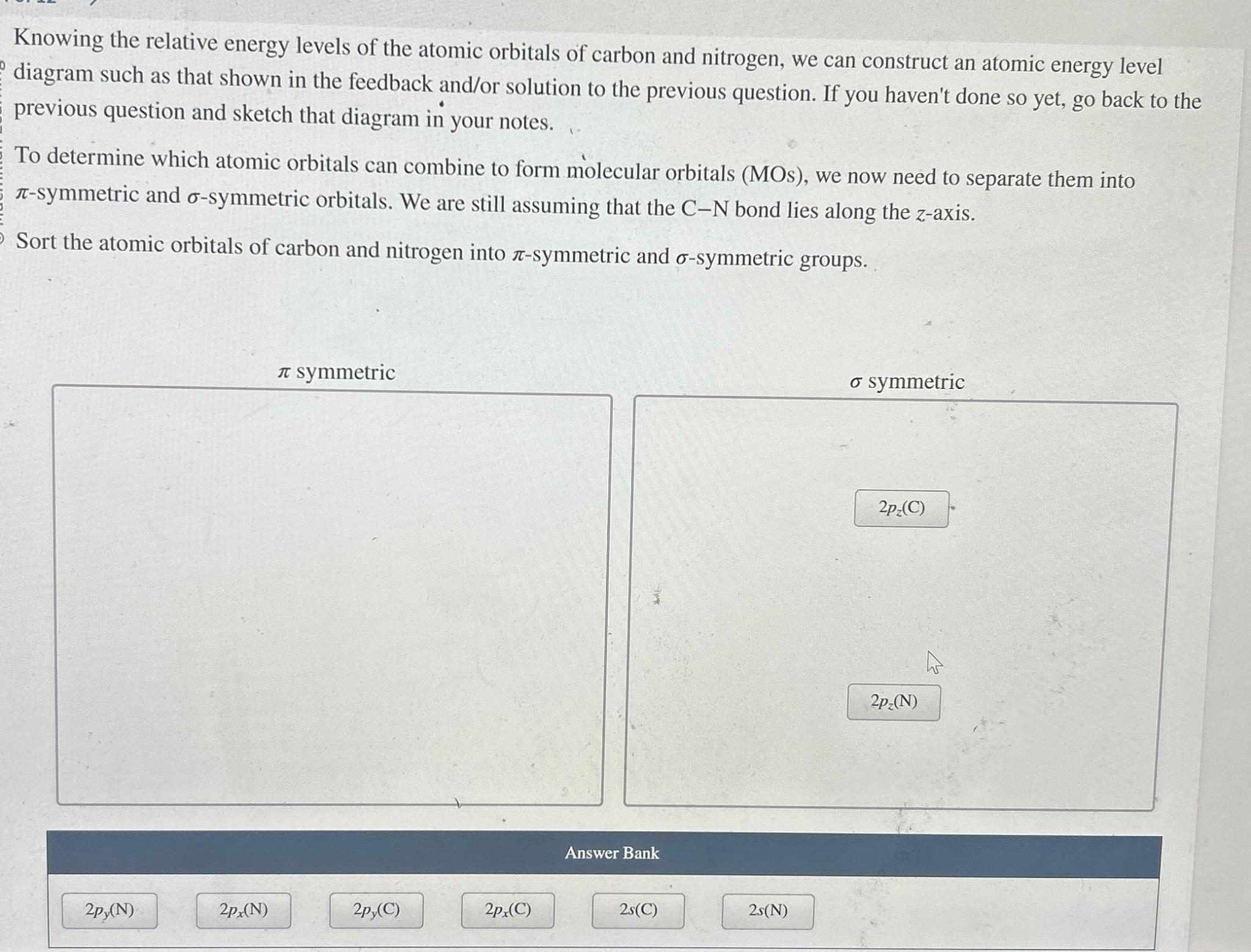
Knowing the relative energy levels of the atomic orbitals of carbon and nitrogen, we can construct an atomic energy level diagram such as that shown in the feedback andor solution to the previous question. If you haven't done so yet, go back to the previous question and sketch that diagram in your notes.
To determine which atomic orbitals can combine to form molecular orbitals MOs we now need to separate them into symmetric and symmetric orbitals. We are still assuming that the bond lies along the axis.
Sort the atomic orbitals of carbon and nitrogen into symmetric and symmetric groups.
svmmetric
symmetric
Answer Bank
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


