Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Lab: The Heat of Dissociation of N2O4 Question I need help on are: 1) If the gases deviate from the ideal gas law, how would
Lab: The Heat of Dissociation of N2O4
Question I need help on are:
1) If the gases deviate from the ideal gas law, how would that affect your estimate of Kp? This would be an example of a systematic error
2)How does the N-N bond energy in N2O4 compare to other N-N bond energies? Can you explain why this bond is weaker than other covalent bonds?
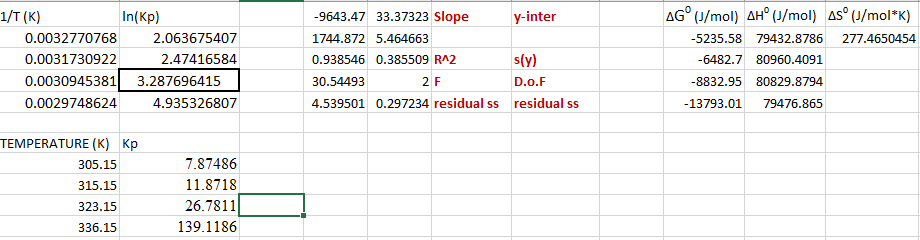
When internal combustion engines burn gasoline, the high temperatures also allow the N2 that is naturally in air to react with O2 to form nitrogen oxides, like NO,N2O, and NO2 as side products. These molecules are all free radicals with high reactivity and toxicity to animals and plants. NO2 has a brown-red color and is principally responsible for the color of smog that plagues Southern California. This molecule can dimerize to form N2O4, a colorless gas that is held together by a weak N-N bond. In this experiment, you will measure the thermodynamic properties of the dissociation reaction: N2O4(g)2NO2(g) including the equilibrium constant Kp, the free energy change G, the enthalpy change H, and the entropy change S. These measurements will allow you to estimate the bond strength of the NN bond in N2O4. Measurements are made by a gas density method (the Dumas method) which requires you to apply the Ideal Gas Law. The free energy relations should be familiar from General Chemistry and Physical Chemistry lecture classes. \begin{tabular}{|r|r|r|r|r|r|r|r|r|r|} \hline 1/T(K) & ln(KP) & 9643.47 & 33.37323 & Slope & y-inter & G0(J/mol) & H0(J/mol) & S0(J/molK) \\ \hline 0.0032770768 & 2.063675407 & & 1744.872 & 5.464663 & 5235.58 & 79432.8786 & 277.4650454 \\ \hline 0.0031730922 & 2.47416584 & & 0.938546 & 0.385509R2 & s(y) & 6482.7 & 80960.4091 & \\ \hline 0.0030945381 & 3.287696415 & & 30.54493 & 2F & D.o.F & 8832.95 & 80829.8794 & \\ \hline 0.0029748624 & 4.935326807 & & 4.539501 & 0.297234 & residual ss & residual ss & 13793.01 & 79476.865 & \\ \hline \end{tabular} TEMPERATURE (K) Kp \begin{tabular}{|r|r|} \hline 305.15 & 7.87486 \\ \hline 315.15 & 11.8718 \\ \hline 323.15 & 26.7811 \\ \hline 336.15 & 139.1186 \\ \hline \end{tabular}Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


